INTRODUCTION
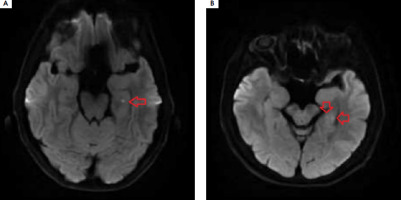
Transient global amnesia (TGA) manifests as acute short-term memory disturbances with a distinctly marked component of anterograde amnesia, which spontaneously resolves within 24 hours, often leaving a gap in memory. Clinical symptoms typically last from 4 to 8 hours, rarely under 60 minutes (9-32%) [1]. The condition is more common in women and primarily occurs in the 6th to 7th decade of life. Diagnosis is based on the 1990 diagnostic criteria of Hodges and Warlow. During an episode, autopsychic orientation is preserved; however, due to impaired encoding of new memories, the patient is disoriented regarding time and place, leading them to ask the same questions repeatedly. No signs of focal damage to the central nervous system are observed. Magnetic resonance imaging (MRI) of the head performed 24 to 96 hours after the onset of symptoms shows minor hyperintense foci in the CA1 sector of one or both hippocampi in over 50% of patients, which are invisible in T2- weighted images and FLAIR sequences, disappearing after 7-10 days (Figure I). The prognosis is good. TGA is usually monophase, but recurrences occur in 2.9-26.3% of cases, with depression and migraines being predisposing factors [1].
Figure I
Typical hyperintense foci in the diffusion-weighted magnetic resonance imaging of head located in the left hippocampus
TGA has a complex and multifactorial background. The pathophysiology of the disease remains unclear. There are numerous theories trying to explain the pathophysiology of TGA, e.g., cortical spreading depression, vasogenic (ischemic), epileptic, psychogenic; however, none of them fully explains this phenomenon [1]. Researchers are continuously trying to identify risk factors for TGA. Werner et al. [2] analyzed the relationship between hypoplasia of the vertebral arteries and the occurrence of TGA. In a study group of 206 patients with acute TGA, it was found that the diameter of the right vertebral artery, measured at the V4 segment, was significantly narrower in the study group than in the control group of healthy individuals without cardiovascular risk factors (2.09 mm vs. 2.35 mm; p < 0.01). No significant differences were noted in the diameters of the left vertebral arteries and internal carotid arteries [2]. Cho et al. [3] analyzed heart rate variability in patients after a TGA episode; they found that the greatest fluctuations occurred during the first week after TGA and decreased over time. Suh et al. [4] conducted volumetric assessment of the brains of 87 patients with TGA based on MRI results, noting greater atrophy of the anterior ventral part of the cingulate gyrus in this group compared to the control group. Rein et al. [5] assessed blood perfusion in a small group of patients (n = 5) during the acute phase of TGA – no patient showed clinically significant ischemia (cerebral blood flow [CBF] < 30%), while 4 out of 5 patients exhibited mild oligemia in areas supplied by the posterior cerebral artery [5]. Noh et al. [6] investigated the relationship between the presence of a patent foramen ovale (PFO) and TGA. This study showed that patients with TGA and PFO had fewer cardiovascular risk factors and fewer chronic vascular changes in the brain compared to a control group. These results suggest that transient global amnesia in patients with PFO may be more related to paradoxical microembolism than ischemia resulting from traditional vascular risk factors. A study by Kawai et al. [7] demonstrated that patients with TGA, compared to a control group of healthy individuals, exhibited a greater biological antioxidant potential in cerebrospinal fluid (csfBAP) and concluded that oxidative stress may be linked to the pathogenesis of TGA. Numerous cases of TGA have been reported in conjunction with other acute medical conditions [8-10], such as takotsubo cardiomyopathy [11], reversible cerebral vasospasm [12], Galen vein thrombosis [13], cardiac myxoma [14], or following unexpected procedures or events [15], such as diving in the ocean [16], administration of an iodinated contrast agent before a computed tomography (CT) scan of the abdomen [17], internal carotid artery stenting [18], or transesophageal echocardiography. High blood pressure (BP) values are often noted during the acute phase of the disease.
This paper presents the case of a patient diagnosed with recurrent TGA, in whom a shortening of coagulation times and high BP peaks were noted during each acute phase of the disease, suggesting transient increased blood coagulability and transient dysregulation of autonomic blood pressure control.
CASE DESCRIPTION
A 59-year-old patient was admitted to the Department of Neurology due to transient short-term memory disturbances lasting approximately 3.5 hours. The patient reported total amnesia of events during that period. The episode most likely occurred while riding a bicycle. The medical history revealed hypertension and migraine with aura. At admission, no abnormalities in neurological examination were noted. High blood pressure was recorded and activated partial thromboplastin time (aPTT) and prothrombin time (PT) were shortened (Table 1). The patient underwent extensive diagnostic evaluations to exclude hypercoagulable states (protein C, protein S, antithrombin III, D-dimers, fibrinogen were normal; no antiphospholipid antibodies were detected in serum nor mutations in the factor V Leiden and the prothrombin G20210A genes). A CT scan of the head and an angio-CT of cerebral vessels showed no significant pathologies. The electroencephalography was normal. Approximately 32 hours after the memory disturbances occurred, an MRI was performed, which revealed two small lesions in the DWI sequence within the left hippocampus. Based on Hodges and Warlow criteria, TGA was diagnosed. Controlled aPTT and PT performed after 4 weeks were normal. After 9 months, the patient was readmitted to the Department of Neurology due to persistent short-term memory disturbances lasting 4 hours. He did not remember what he had done in the last 24 hours. Neurological examination revealed no focal symptoms, he was asking the same questions every 30 seconds, being confused about time, but correctly oriented in terms of autopsychic awareness. High blood pressure values of 180/109 mmHg were noted again. Laboratory tests once again showed shortened aPTT and PT, which gradually normalized over the following days (Table 1). The patient was not taking any medications that affect blood clotting. The CT and MRI of the head, as well as video EEG, were normal. On the third day of hospitalization, an ambulatory blood pressure monitoring was performed, revealing exceeded blood pressure values during the day and at night; however, the highest recorded values were significantly lower than those at admission (Table 1). Clinical symptoms resolved within 24 hours, leaving an approximately 8-hour gap in memory. Another episode of TGA was diagnosed, and antihypertensive treatment was intensified.
Table 1
Evolution of laboratory values of blood coagulation parameters and blood pressure
COMMENT
To the best of the authors’ knowledge, this is the first case report of recurrent TGA consistently associated with transient shortening of aPTT and PT. After the first episode of TGA, the result was considered a preanalytical error. Upon TGA recurrence and again noting the shortening of coagulation times, serial measurements were performed over the following days until normalization. Hypercoagulation conditions were excluded.
Shortened aPTT often results from a preanalytical error (e.g., issues with blood sample collection, improper storage) and PT from excessive vitamin K supplementation [19]; however, they may also result from a prothrombotic state (e.g., inflammation) due to activation of the intrinsic (aPTT) or extrinsic (PT) coagulation pathway. A relationship between shortened aPTT and strong emotional stress has been described [20, 21], and it has also been recognized as a factor worsening the prognosis of ischemic stroke [22].
TGA is often associated with a mental or psychical stress preceding the onset of symptoms, and a stress response can influence coagulation and blood pressure values. When the TGA episode is triggered by a stressful event, an acute phase reaction can potentially lead to shortened clotting times (increased clotting factors) and dysregulation of the central blood pressure control. TGA, coagulation disorders and blood pressure abnormalities may possibly share a common underlying cause, and in cases where coagulation disorders and blood pressure abnormalities precede TGA, they may serve as potential trigger factors for TGA.
During TGA, autonomic nervous system dysfunction occurs (e.g., high BP peaks, heart rhythm disturbances) and the occurrence of symptoms is often preceded by increased physical exercise or mental stressor [1, 23]. Seasonality of the occurrence has also been demonstrated [24].
Therefore, a relationship between the stressful factor, acute autonomic dysfunction and the occurrence of TGA is possible. The shortening of aPTT and PT may suggest a relationship between TGA and transient blood coagulation disorders in the acute phase. Further studies are necessary to better understand this relationship.







