INTRODUCTION
Drug reaction with eosinophilia and systemic symptoms (DRESS syndrome), also known as druginduced hypersensitivity syndrome (DIHS), is a rare, severe, potentially life-threatening disorder that might affect internal organs. DRESS differs from other drug eruptions by late onset and the possible persistence or worsening even after drug discontinuation [1]. Clinical presentation of DRESS encompasses a wide spectrum of symptoms, including cutaneous eruptions such as a maculopapular rash, fever, lymphadenopathy, hematological abnormalities (e.g. eosinophilia and atypical lymphocytes), facial edema, and multi-organ involvement affecting the liver, kidneys, lungs, pancreas, heart, and other organs.
Compared to other cutaneous adverse drug reactions, DRESS has a delayed onset, with initial symptoms typically appearing 2 to 8 weeks after treatment initiation, although this timeframe may vary depending on the specific drug administered. Having been reexposed to the drug, the patient develops symptoms more rapidly. The most common causes of DRESS are aromatic antiepileptic agents, allopurinol, antibiotics, such as sulphonamides and vancomycin. However, a wide range of drugs have been reported as a possible causative factor [2–4]. As an aromatic anticonvulsant, lamotrigine is among the drugs most frequently associated with DRESS syndrome, with an estimated incidence of 1 in 10,000 to 1 in 1,000 among patients treated with anticonvulsants [5]. Conversely, azithromycin, a widely prescribed macrolide antibiotic, is an atypical inducer of DRESS as only a few cases have been described in the literature with symptoms onset occurring shortly after the drug administration [6, 7]. Given the widespread use of antibiotics and rising prevalence of polypharmacy, prompt recognition of DRESS syndrome is of utmost importance.
OBJECTIVE
The aim of the article is to outline two cases of successfully treated DRESS syndrome, the first one induced by lamotrigine and the other by azithromycin in order to highlight the importance of rapid diagnosis and management of this condition, particularly in the era of rising polypharmacy.
CASE REPORTS
Case 1
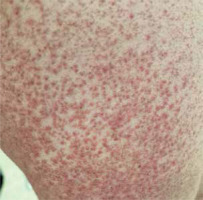
An 18-year-old patient was referred to the hospital with a history of generalized confluent maculopapular eruption affecting the face, chest and limbs accompanied by fever, lymphadenopathy, facial edema and overall poor well-being. The patient has suffered from arthrogryposis since early childhood. Additionally, she remains under constant psychiatric care because of depressive disorders and prior suicidal attempts, treated initially with sertraline. She denied any adverse drug and allergic reactions. Family history revealed psoriasis in her father. Prior to hospital admission, due to the mood disorders, there was a modification of the patient’s chronic psychiatric therapy. For the first time she was prescribed an anticonvulsive drug – lamotrigine initially at a dose of 25 mg/day, gradually increased to 75 mg/day. After 3 weeks of medication, she developed a macular rash on the forearms, which rapidly progressed to generalized maculopapular lesions with periorbital edema, accompanied by pruritus and burning sensation. Thus, she was prescribed oral antihistamines by her GP. Despite a brief improvement, her symptoms relapsed 2 days after discontinuing the medication. She subsequently presented with nausea and a fever of 39°C. She was immediately admitted to the Dermatology Department for further evaluation and management. Upon admission lamotrigine was discontinued as it was strongly suspected to be the causative factor. On physical examination, generalized, confluent maculopapular rash affecting predominantly the face, chest and upper limbs was noted (figs. 1, 2). She had mild non-tender submandibular and postauricular lymphadenopathy as well as slight facial edema.
Blood tests revealed an elevated level of C-reactive protein 72 mg/l (N < 5 mg/l), slight eosinophilia 0.78 G/l (N 0.03–0.27), atypical lymphocytes, increased liver function, with aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels of 72 U/l and 45 U/l, respectively (normal < 35 U/l). Blood cultures, antinuclear antibodies (ANA-4), viral screening including HBV, HCV, EBV, CMV were all negative. There were no abnormalities in chest radiograph. Abdominal ultrasonography revealed splenomegaly. Skin biopsy showed lymphocytic inflammatory infiltrate within superficial dermis and around the vessels. Over the following days, laboratory tests revealed increasing ALT/AST up to 226/118 U/l and GGTP reaching 222 U/l (N < 38 U/l). Based on clinical presentation, medical history and laboratory abnormalities, the patient was diagnosed with DRESS syndrome, secondary to recently introduced lamotrigine. The onset of her symptoms occurred within 3 weeks of starting lamotrigine. During her 2-week hospital stay, corticosteroids were administered; initially intramuscularly (dexamethasone 8 mg), followed by oral prednisolone (25 mg daily), together with antihistamines and topical corticosteroids, resulting in gradual resolution and darkening of the skin lesions. After discharge, she was recommended to continue oral prednisolone 20 mg daily with gradual tapering; the dosage was reduced by 5 mg every 5 days. On her follow-up visit after 2 months in the outpatient clinic, her rash resolved completely, and lab tests were within norms, indicating complete recovery.
Case 2
A 53-year-old woman presented to the hospital emergency department (ED) due to diffuse maculopapular rash with fever, dyspnea and cough. Her medical history was notable for insulin resistance and a previous sinus surgery. Two weeks prior to hospitalization, she had reported to her primary care physician due to otitis symptoms. She was prescribed azithromycin. Subsequently, pruritic skin lesions appeared on the anterior surfaces of the thighs on the second day of antibiotic therapy. Over the next 4 days, the rash steadily progressed, ultimately prompting the patient to discontinue the antibiotic. In addition to the progressing rash, she developed a fever of 40°C, cough, nausea, and diarrhea, which led her to call the Emergency Medical Team. In ED, after a CT scan of the chest, the suspicion of community-acquired pneumonia was confirmed. On admission to the internal disease ward, she was in moderate clinical condition, without fever but presented with a dry cough and resting dyspnea. She required oxygen therapy. Upon physical examination, the patient had a severe, pruritic maculopapular rash affecting 90% of the BSA, covering the trunk and limbs and extending to the lower limbs (figs. 3, 4). On her palms the presence of erythematous macules resembling target lesions was noted. Mucosal areas were spared and there was no lymphadenopathy. Auscultation revealed wheezes. Upon admission, she received intravenous ceftriaxone and ciprofloxacin. Initial laboratory tests revealed leukocytosis WBC count 21.2 G/l (N 4.0–10 G/l), neutrophilia 16.7 G/l (N 2.0–7 G/l), eosinophilia 2.86 G/l (N 0.03–0.27 G/l), atypical lymphocytes, increased C-reactive protein 104 mg/l, and procalcitonin 1.34 ng/ml (N < 0.5 mg/ml). Moreover, elevated liver enzymes were detected: AST and ALT, 321 U/l and 48 U/l, respectively, GGTP 131 U/l (N < 38 U/l), alkaline phosphatase (ALP) 294 U/l. EBV IgG antibodies were positive. Her blood culture, viral hepatitis screening were all unremarkable. Abdominal ultrasonography showed no abnormalities. After receiving the sputum culture result: indicating complete recovery. Escherichia coli ESBL (+), ceftriaxone and ciprofloxacin were discontinued and Tazocin was initiated. Owing to her medical history and clinical picture, she was diagnosed with DRESS syndrome induced by azithromycin. Therefore, she remained under constant dermatological supervision. She was started on systemic steroid treatment that initially included dexamethasone, substituted by pulse therapy with a high dose of intravenous methylprednisolone (30 mg/kg/day) administered for 3 days, followed by prednisone at a dose of 40 mg daily reduced by 5 mg every 10 days resulting in subsiding lesions with scaling, improvement in blood test results as well as the patient’s general condition. She was discharged on oral prednisone at 30 mg daily and scheduled for a follow-up visit in 2 months, at which complete resolution of her symptoms was observed.
DISCUSSION
DRESS syndrome is a severe, potentially life-threatening adverse drug reaction with a mortality rate up to 10%. This article presents 2 cases of successfully treated DRESS syndrome secondary to an anticonvulsant drug and antibiotic. Classified as a delayed hypersensitivity reaction, DRESS typically manifests 2–8 weeks after the first administration of the new drug. The pathogenesis of DRESS has not yet been firmly established, however, a combination of immune system activation, genetic predispositions and viral reactivation play a pivotal role [8]. Certain drugs act as haptens, binding to proteins and triggering an immune response, while reactive drug metabolites can directly damage cells, inducing T-cell activation. There is plausible evidence of the reactivation of viruses, particularly human herpesvirus 6 (HHV-6) which has been suggested as the most frequent potential diagnostic marker for DRESS. The literature also highlights HHV-7, Epstein-Barr virus (EBV) and cytomegalovirus (CMV) reactivation in DRESS pathogenesis [2, 9–11].
Anticonvulsant drugs have been proven to be a causative factor, with an estimated incidence of 1 in 1,000 patients, particularly during the initiation of the therapy or dose escalation [5]. This correlates with the first case, where symptoms developed 3 weeks after commencing lamotrigine. Whereas azithromycin-induced DRESS is extremely rare, with only a few cases reported in the literature. Sharifzadeh et al. in their 2020 review researched 254 instances of antibioticinduced DRESS, two of which (accounting for 0.79% of all cases) occurred rapidly following azithromycin administration bearing resemblance to our case [6]. Additionally, Sriratanaviriyakul et al. documented the case of azithromycin-induced DRESS similar to septic shock [7]. The second case pinpoints the need for acknowledgement of atypical triggers, namely macrolide antibiotics, which are widely prescribed and often underestimated as causative agents for hypersensitivity reactions. The clinical picture of DRESS is diverse and is characterized by a combination of symptoms such as cutaneous skin eruptions, fever, hematological abnormalities such as eosinophilia or atypical lymphocytes and systemic involvement [3]. Owing to unremarkable viral and autoimmune laboratory tests, other potential diseases were ruled out.
Pulmonary manifestations in DRESS syndrome are less common but possible, typically presenting as interstitial pneumonitis, pneumonia or, in severe cases, acute respiratory distress syndrome (ARDS) [12]. In the second case described, the patient experienced respiratory symptoms, and sputum cultures revealed Escherichia coli ESBL (+), strongly suggesting bacterial pneumonia rather than pulmonary involvement directly associated with DRESS.
The RegiSCAR (Registry of Severe Cutaneous Adverse Reactions) scoring system is a frequently applied diagnostic tool [13] and includes following criteria (table 1). Both cases in this report achieved a score of 6 points, confirming a definitive diagnosis of DRESS syndrome.
Table 1
RegiSCAR scoring system for diagnosing DRESS syndrome [14]
The basis of the management is prompt withdrawal of the suspected offending drug. Clinical symptoms may paradoxically deteriorate few days after discontinuation of the causative drug [14]. Systemic corticosteroids are considered to be a first-line treatment of DRESS syndrome at the acute phase and the dose should be gradually reduced over 6–8 weeks in order to prevent the relapse of symptoms [4, 10]. Both patients in this report responded favorably to corticosteroids, with complete resolution of symptoms and normalization of laboratory tests on follow-up visits.
CONCLUSIONS
Because of its complex etiology and diverse clinical picture, DRESS syndrome remains a diagnostic and therapeutic challenge. Early identification of DRESS, including discontinuation of the culprit drug as well as initiation of systemic corticosteroids are essential to prevent severe complications. Since the simultaneous over-prescription of medications has become a commonplace practice, the possibility of developing drug-induced hypersensitivity reactions is on the rise. Greater awareness of common and atypical causative factors is necessary in order to implement appropriate care and achieve desirable medical outcomes.