Introduction
The management of patients with acute surgical pathologies complicated by peritonitis in the early postoperative period is an urgent problem in modern abdominal surgery [1]. After surgery on the abdominal organs, patients often have a muscle tone imbalance resulting from functional or organic lesions of innervation. There is also inhibition of the motor activity of the gastrointestinal tract, in which there is an accumulation and delay in the discharge of gases and stool [2]. At the same time, patients have an absence of peristaltic noises or a noticeable depression being listened to and an increase in abdominal volume. In the literature [3], paresis is also called functional intestinal obstruction, postoperative flatulence, dynamic intestinal obstruction, paralytic intestinal obstruction, or functional stasis [4]. Paresis is one of the most common postoperative complications [5]. A promising measure for the prevention of postoperative paresis is the early start of enteral (tube) nutrition, which contributes to a more rapid recovery of functional activity [6]. Despite significant advances in modern surgery and anaesthesiology, the frequency of postoperative paresis in patients is 10–17% [7]. According to other data, the frequency of postoperative complications accompanied by paresis varies between 5% and 50% [8].
To date, there is no single approach to the treatment of this pathology in clinical practice. At the same time, it is important to identify early signs of paresis and choose effective methods of treating patients. In abdominal surgery, intestinal drainage is widely practised to prevent the development of postoperative paresis. Methods of intestinal drainage may be non-surgical and operational. Non-surgical methods include drainage of the small intestine using Miller-Abbott type probes [9]. Also, nasoenteral endoscopic intubation and transrectal decompression intubation of the large intestine are performed [10].
Surgical methods of drainage of the small intestine are closed, open, and combined [11]. To carry out seal drainage, opening the lumen of the hollow organs of the gastrointestinal tract is not required [12]. During opencut drainage, artificial fistulas are formed in the stomach or intestines [13]. Combined drainage methods combine, at the same time, separate techniques characteristic of closed and open methods [14]. Closed drainage methods, in turn, include nasoenteral drainage and transrectal intubation of the small intestine [15].
Currently, preference is given to methods of drainage of the gastrointestinal tract, in which, in addition to passive emptying, active electrical stimulation of the gastrointestinal tract is performed. Electrostimulation of the gastrointestinal tract, similar to stimulation of the human heart, is very promising in modern medicine. This is because the organs of the gastrointestinal tract, as well as the heart, have their electrical signals, which can be changed by supplying certain types of electric currents from the outside, through intraluminal or serous electrodes, to certain areas of the gastrointestinal tract. Many studies have shown good results in using the method of electrical stimulation for the treatment of various diseases, including intestinal paresis, dumping, and short bowel syndrome [16].
To date, electrical stimulation of the gastrointestinal tract is attracting increasing attention from researchers and clinicians. As the opportunity arises of introducing new methods and promising results achieved in the treatment of paresis of the stomach and intestines. Known methods of electrostimulation of the gastrointestinal tract include performing laparotomy to eliminate the cause of peritonitis or intestinal obstruction and sanitation of the abdominal cavity. After that, a 2-light probe is inserted transnasally into the gastrointestinal tract, on the wall of which electrodes are located along the entire length at a distance of 20–25 cm from each other. Electrical stimulation of the intestine begins in the 3 h following surgery. By consistently increasing the current strength, the threshold of intestinal excitability is determined, at which maximum spontaneous electrical activity appears. Subsequently, with this current strength, the intestine is stimulated by a series of monopolar pulses. The disadvantage of this method is the simultaneous action of current in the area of the location of all the electrodes, which excludes the possibility of creating successive peristaltic waves [17]. Selective stimulation of the small or large intestine is also impossible [18]. Despite some disadvantages of electrostimulation of the gastrointestinal tract, this method is widely used in the clinic and is constantly being improved.
In addition to correcting postoperative paresis, it is important to predict this condition. One study showed a relationship between variants of the SERT gene (the serotonin transporter) regulating serotonin reuptake and its concentration in blood plasma, as well as the likelihood of postoperative intestinal paresis. These data will allow not only prediction of the occurrence of postoperative disorders of the motor-evacuation function of the intestine but also improvement of the algorithms of prevention and treatment based on pathogenesis [19]. Therefore, it is important not only to correct paresis but also to predict this condition, for which it is necessary to search for informative methods.
Because each method has both advantages and disadvantages, there is a need to develop reliable, accessible methods for the prevention and treatment of postoperative intestinal paresis for practitioners. The outcome of the operation and the viability of intestinal anastomoses depend on how quickly the problem of postoperative intestinal paresis can be solved [20].
The high incidence of intestinal paresis in the early postoperative period and the danger of this pathology are current problems.
Aim
The purpose is to study the informativeness of the methodology for determining the risk of developing postoperative intestinal paresis and the effectiveness of using a specially developed method for restoring intestinal motility through electrical stimulation. Following the purpose of the study, the following tasks were set:
To diagnose acute surgical pathologies in patients who have been admitted to the clinic.
To predict the probability of developing postoperative intestinal paresis in patients with acute surgical pathologies complicated by peritonitis according to the proposed method by calculating the temperature coefficient.
To evaluate the effectiveness of the developed method of electrostimulation of the gastrointestinal tract to restore intestinal motility in patients with a high risk of postoperative paresis.
Material and methods
The study was conducted on 20 patients who were admitted to the Rahat clinic in Almaty (Kazakhstan) with acute surgical pathologies complicated by peritonitis. The basis for the application of the method of electrostimulation of the gastrointestinal tract was the result of the preoperative prognosis. For this purpose, a method was used to predict the occurrence of postoperative intestinal paresis, developed by V.V. Brachevoy. According to this technique, the initial temperature values of the mucous membrane and the skin of the cheek were measured in patients, after which the difference between these values (t1) was calculated. Further, patients took xanthinol nicotinate orally on an empty stomach at a dose of 0.3 g (2 tablets). This drug, along with other nicotinic acid derivatives (theonicol, complamine), is used to activate fibrinolysis [21]. The dose is selected individually and, as a rule, it is 0.3–0.6 g per day [22].
After 15–20 min, the temperature difference between the mucous membrane and the cheek skin was measured again in patients (t2). Then the temperature coefficient (T) was calculated using the following formula: T = t1 – t2 (1), where: t1 is the difference between the temperature of the mucous membrane and the skin of the cheek of patients before taking xanthinol nicotinate, and t2 is the difference between the temperature of the mucous membrane and the skin of the cheek of patients 15–20 min after taking xanthinol nicotinate.
Based on the temperature coefficient indicators, the development of postoperative atony was predicted. At T ≥ 1, the development of postoperative atony was considered unlikely. This method of predicting postoperative atony is easy to perform and is widely used in clinical practice [23].
In the case of a significant probability of developing postoperative intestinal paresis during surgery, a 2-light probe with 2 groups of electrodes located on its surface was inserted along the entire length of the duodenum and small intestine. One part of the probe, 2 m long, had perforations along its entire length, providing decompression of the intestine. The other half of the probe had no holes, but wires passed through it to each of the 6 electrodes separately. Electrostimulation was performed in the 3 h after the operation, supplying current selectively to the proximal or distal group of electrodes, while aspirating the contents of the intestine. Two groups of hollow electrodes were installed on the probe wall, which are steel cylinders with a diameter of 1.2 cm and a length of 0.8 cm. Each group of electrodes consisted of 3 electrodes located at a distance of 10–12 cm from each other. The distance between the individual groups of electrodes was 135 cm. The placement of electrodes at such a distance provides selective stimulation of the gastrointestinal tract in the pacemaker zones located in the pyloroduodenal department and the zone of the ileocecal transition of the gastrointestinal tract. Also, it contributes to the formation of isoperistaltic contractions of the smooth muscles of the intestine not only in the area of exposure to the electrodes but also throughout the action of the gastrointestinal pacemakers [24].
Thus, stimulation in the area of the pyloroduodenal junction leads to the appearance of successive isoperistaltic waves in the outlet of the stomach, duodenum, and small intestine. Electrical stimulation in the ileocecal region leads to the occurrence of isoperistaltic contractions of the ileocecal valve, the cecum, and the colon up to the rectum. Moreover, the strength of smooth muscle contractions remains the same regardless of the distance of the electrodes, which allows for stimulation without resorting to the action of electrodes on the rest of the gastrointestinal tract [18].
To stimulate the intestine with the help of an enterography, the gastrointestinal tract, which was in a state of paresis (small or large intestine), was determined. The stimulation was carried out for 30 min. Next, dynamic control of intestinal motility recovery was carried out 2, 4, and 6 h after an electrostimulation session using enterography. If, during this time, after a temporary increase in motor skills, its decrease was recorded, then electrical stimulation was repeated for 30 min with the same current strength. In such cases, the probe was removed after 3–4 days, after the restoration of persistent intestinal motility. If the restoration of intestinal function took place earlier, the probe was removed after 1–2 days. Withdrawal of gases, stools, etc. was considered a sign of recovery of intestinal peristalsis in patients [25].
Results
Patients were admitted to the clinic with acute surgical pathologies complicated by peritonitis. 30% were diagnosed with acute appendicitis, 25% with acute cholecystitis, 20% with acute intestinal obstruction, 15% with perforated gastric ulcer and duodenum, and 10% with a strangulated hernia of the abdomen (Figure 1).
Figure 1
Acute surgical pathologies complicated by peritonitis: 1 – acute appendicitis, 2 – acute intestinal obstruction, 3 – acute cholecystitis, 4 – perforated stomach ulcer and 12 duodenum, 5 – pinched abdominal hernia
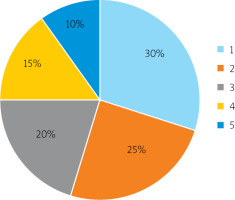
Thus, all the examined patients had acute surgical pathologies complicated by peritonitis, including appendicitis, intestinal obstruction, cholecystitis, perforated gastric ulcer and duodenal ulcer, and strangulated abdominal hernia.
According to current practical recommendations, patients with peritonitis should undergo emergency surgery; however, treatment algorithms may differ in each case [26]. The degree of intraperitoneal infection and the associated systemic inflammatory response are the main determinants of the severity of secondary peritonitis, which should be taken into account when making therapeutic decisions. Despite the wide range of available laboratory and radiographic tests and their usefulness for assessing abdominal pain, secondary peritonitis remains primarily a clinical diagnosis. Laboratory testing is not enough to determine which patients need surgery or are at risk of perforation due to suffocation or mesentery ischaemia. The management of secondary peritonitis has undergone significant changes due to the improvement of percutaneous drainage under visual control and laparoscopy [27].
In this study, all patients with the above acute surgical pathologies were shown intestinal intubation [28]. Before carrying out this procedure, a temperature coefficient was calculated in patients, the values of which served as the basis for predicting the likelihood of developing postoperative intestinal paresis (Table I).
Table I
Development forecast of postoperative intestinal paresis
[i] T is the temperature coefficient (T= t1 – t2); t1 is the difference between the temperature of the mucous membrane and the skin of the cheek of patients before taking xanthinol nicotinate; t2 is the difference between the temperature of the mucous membrane and the skin of the cheek of patients 15–20 min after taking xanthinol nicotinate.
Thus, out of 20 examined patients, 15 had a low probability of developing postoperative intestinal paresis (75%) and 5 (25%) had a high risk of developing this complication (Figure 2).
Figure 2
Prognosis of postoperative intestinal paresis in patients: 1 – low probability of complications, 2 – high probability of complications
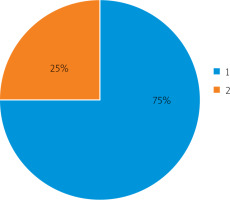
Other sources note that the following factors may contribute to the occurrence of intestinal paresis: operations for diffuse peritonitis [29]; destructive pancreatitis, severe intestinal obstruction; Leriche syndrome [30]; abdominal aortic aneurysm; operations on mesentery vessels, inferior vena cava, spine, or ureters; plastic surgery on the anterior abdominal wall to reduce abdominal volume, etc. [31]. In addition, the development of paresis can be triggered by concomitant pathologies: diabetes mellitus, hypertension, the presence of occlusions of intestinal vessels, chronic gastrointestinal diseases, or dysbiosis [32, 33]. Also, intestinal paresis has a high risk of developing in the elderly, and in those presenting with overweight [34, 35]. So, these factors are used in predicting the risk of developing postoperative intestinal paresis.
Other methods of predicting the risk of developing intestinal paresis are also used in surgical practice. Therefore, in one study, the factors of postoperative intestinal paresis in patients who underwent colon surgery were determined. The study involved 313 patients who underwent colon surgery. The comparison group consisted of 152 patients in whom prevention and treatment of intestinal paresis were carried out by conventional methods. In the main group (161 patients), improved methods were used to treat paresis, and prognostic risk factors for the occurrence of this pathology were also taken into account. To monitor the motor-evacuation function of the gastrointestinal tract before and after surgery, a method was developed based on electrogastroenterography (EGEG) using a microprocessor – EGEG-MP03. Along with other electrophysiological methods for assessing the motor-evacuation function of the gastrointestinal tract, EGEG is being introduced into modern clinical practice and is a promising direction that allows for the detection of disorders and improvement of treatment methods [36]. The patients were examined before the operation, 12 h after the operation, at the end of the 1st day, and on the 2nd, 3rd, 4th, and 5th days after the operation. Special electrodes were applied to the limbs of patients and in the umbilical zone. The study was conducted for 60 min. EGEG signals were analysed using wavelet transformations, which made it possible to decompose the original signal by frequency while fixing the appearance of this frequency in time [37].
Based on the study of the clinical picture of the disease and the results of monitoring of intestinal motility of patients, risk factors for gastrointestinal paresis in the early postoperative period were identified [38]:
male,
concomitant pathologies,
age over 60 years,
surgical interference over 2 h,
combined surgical interventions,
adhesions in the abdominal cavity requiring intraoperative viscerolysis,
intraoperative blood loss of more than 1 l,
gastrointestinal dysmotility according to EGEG (stenotic lesions of the colon without clinical signs of intestinal obstruction),
clinical signs of partial intestinal obstruction before surgery.
The presence/absence of factors was noted in each patient, and the score was calculated accordingly, after which the probability of developing paresis was predicted. In the comparison group, among 152 patients, 80 had no more than 3.5 points, while intestinal paresis was found in 1 (1.25%) patient only. In the observations, the total score was 4–5.5, while intestinal paresis occurred in 21 out of 34 (61.8%) patients after surgery, and with a total score of 6 or more, intestinal dysmotility was observed in 36 out of 38 (94.7%) patients. The prognostic assessment of the risk of developing intestinal paresis was more reliable when assessing the EGEG indicators, which quite often outstripped the clinical manifestations of postoperative intestinal paresis. Due to the high informativeness, EGEG indicators can be used to correct treatment in the early stages of the postoperative period until signs of intestinal dysfunction appear [37].
Because a high risk of developing postoperative intestinal paresis was predicted in 5 patients in this study, electrostimulation of the gastrointestinal tract was performed on the last one. After this procedure, the effectiveness of therapy was evaluated in the patients (Table II). Two patients were diagnosed with acute gangrenous perforated appendicitis complicated by diffuse purulent peritonitis. With the help of the proposed method of electrostimulation of the gastrointestinal tract, it was possible to achieve stable results of therapy on the first day, after which the probe was removed (Table II).
Table II
Application results of the developed electrical stimulation method of the gastrointestinal tract
Discussion
Other studies confirm that intestinal intubation is one of the most effective methods of prevention and treatment of postoperative gastrointestinal paresis. At the same time, it is noted that the prevention and treatment of this pathology must be combined with complex therapy, which contributes to the correction of homeostasis disorders in the patient’s body. At the same time, measures to prevent the development of paresis should be started before the surgical intervention. Many studies have noted that the motor-evacuation function of the intestine is better restored after surgery if circulatory disorders and gastrointestinal hypoxia, and hypokalaemia are eliminated and the intestines are cleared of stagnant contents [39–41]. Therefore, there are various ways to prevent the development of postoperative intestinal paresis, which are important to apply before surgery. To restore gastrointestinal peristalsis after surgery, it is recommended that the tone of the sympathetic nervous system be reduced (for example, using benzohexonium), and an infusion of polyionic solutions be conducted based on polyatomic alcohols (Sorbilact, Reosorbilact, glucsil, xylate) and prokinetics and β-adrenolytics be prescribed to patients.
In this study, the developed gastrointestinal electrostimulation method gave a reliable result of improved intestinal motility in 1 patient with acute adhesive strangulation obstruction of the small intestine, gangrene, and diffuse purulent peritonitis on the first day, which allowed the probe to be removed (Table II). One patient with a pinched oblique inguinal-scrotal hernia and gangrene of the small intestine also had a postoperative complication in the form of diffuse purulent peritonitis. The proposed gastrointestinal electrostimulation method made it possible to restore intestinal peristalsis on the first day of therapy, after which the probe was removed (Table II). One patient was diagnosed with acute adhesive strangulation small intestinal obstruction, after which there was development of gangrene of the small intestine and diffuse purulent peritonitis. The developed gastrointestinal electrostimulation method gave a weak result on the first day but contributed to the complete resumption of intestinal motility on the second day, after which the probe was removed (Table II).
It should be noted that the 2 patients had different treatment results in this study, although the postoperative diagnosis was the same: acute adhesive strangulation small bowel obstruction, gangrene of the small intestine, as well as diffuse purulent peritonitis (Table II). In 1 case, the probe was removed on the first day, and in the second it was removed on the second day, when there was a complete restoration of intestinal motility. In general, regardless of the type of postoperative complications, the proposed method of electrostimulation of the gastrointestinal tract showed high efficiency in all patients.
Electrostimulation of the gastrointestinal tract in patients with a predicted high risk of postoperative paresis by the proposed method made it possible to completely restore intestinal peristalsis on the first day in 4 (80%) patients and on the second day in 1 (20%) patient (Figure 3).
Figure 3
The terms of complete restoration of intestinal motility after the application of the proposed gastrointestinal electrostimulation method in patients: 1 – on the first day, 2 – on the second day
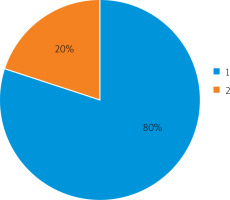
Therefore, the proposed gastrointestinal electrostimulation method showed high efficiency in this study in terms of the resumption of intestinal motor function in patients at risk of developing postoperative intestinal paresis. At the same time, the majority of patients (80%) had a complete recovery of the motor-evacuation function of the intestine on the first day and some patients (20%) on the second day.
In another study, the method of intraluminal electrostimulation of the pacemaker of the small intestine was tested in the treatment of patients with postoperative paresis. Restoration of the motor-evacuation function of the intestine after surgery was performed using a gastrointestinal probe containing electrodes. The study involved 41 patients aged 15 to 82 years, who developed purulent peritonitis and acute intestinal obstruction after surgery. Of these, 11 patients were with an adhesive disease, 7 with strangulated hernias, 6 with perforated duodenal ulcer, 7 with abdominal wounds, 5 with colon tumours complicated by intestinal obstruction, and 5 with gangrenous appendicitis. The severity of peritonitis was assessed based on the Manheimer peritonitis index on an 8-factor risk scale [42].
Patients of the clinical comparison group aged 17–89 years with the same pathologies underwent standard intestinal intubation. All patients underwent surgery and were prescribed standard drug therapy. Evaluation of the effectiveness of intraluminal electrical stimulation of the intestine was carried out according to the main clinical criteria for restoring intestinal motility, as well as objective registration of the dynamics of bioelectric activity of the gastrointestinal tract. Electroenterography was performed on a modified EGS-4m electrogastrograph using an active frequency filter that operated in the range 0.2–0.4 Hz, which corresponds to the electrical activity of the small intestine [43]. The active electrode was installed at a standard point 4 cm down from the xiphoid process and 4 cm to the right of the midline. Graphic registration of electrical activity was performed in several stages: upon admission of the patient to the department, 12 h after surgery, and every day until the restoration of stable intestinal function. The study was conducted for 10 min. The results of the enterograms were evaluated by the amplitude of the waves, and the average amplitude and power were calculated.
It was noted that the intraluminal electrostimulation method of the small intestine pacemaker made it possible to rapidly achieve the resumption of intestinal peristalsis, preventing the development of paresis. Electrostimulation generally shows high efficiency and prevents repeated surgical interventions in combination with other therapeutic measures, especially with prolonged epidural anaesthesia. If electrical stimulation is ineffective, it indicates the presence of an ongoing pathological process in the abdominal cavity and can be used as a criterion for the effectiveness of therapeutic measures, diagnostics, and indications for repeated laparotomy. If the immediate postoperative period is favourable, electrical stimulation has a positive effect after 2 sessions of active stimulation.
The fastest and most reliable effect can be achieved if electrical stimulation is performed at the end of the second or the beginning of the third day after surgery. If peritonitis slowly regresses, stimulated intestinal motility gradually decreases; therefore, to maintain the effect, it is necessary to repeatedly repeat electrical stimulation sessions. Electroenterograms recorded from the body surface reflect the state of intestinal peristalsis, which makes it possible to monitor the immediate postoperative period [44].
According to the results of the study, it was shown that early stimulation could significantly reduce the period needed to restore the motor-evacuation function of the intestine. Optimal intestinal motility was restored in 22% of cases. Among patients who did not undergo electrostimulating therapy, at the end of the second day, this indicator was significantly higher: 86% in patients who received electrostimulation. Therefore, intraluminal electrical stimulation of the gastrointestinal tract is effective both in the complex therapy of patients with postoperative paresis and peritonitis, and in the prevention of gastrointestinal paresis after surgery. Restoration of the motor-evacuation function of the gastrointestinal tract in patients who received intraluminal electrical stimulation mainly took place at the beginning of the second day after surgery. In the group of patients who did not undergo electrical stimulation, normalization of intestinal motility was manifested on the third day. The use of the electrostimulation method made it possible to reduce the duration of probe intestinal decompression by 1 day. In general, the developed method of restoring intestinal peristalsis and the device for its implementation are recognized as effective in the treatment of patients with postoperative intestinal paresis, according to the results of the clinical study [45].
Another study conducted a systematic review to evaluate the effectiveness of gastrointestinal electrostimulation methods in patients with postoperative intestinal paresis. A critical review was compiled covering all included studies, as well as evaluation and synthesis of stimulation methods, protocols, and clinical results. A wide range of neuromodulation strategies and protocols were identified and evaluated. Improvement of postoperative recovery of the gastrointestinal tract after electrostimulation was reported by 55% of studies (10/18), most often those that evaluated percutaneous electrostimulation and electroacupuncture therapy (7/10). Several studies reported a reduction in the time of withdrawal of the first gases and stool, a shorter duration of hospital stay, and a decrease in the severity of postoperative pain. However, inconsistent reporting and limitations in the test model did not allow us to determine the effectiveness of electrical stimulation definitively. It was noted that electrostimulation seemed to be a promising method for postoperative restoration of gastrointestinal function. In further studies, it is planned to apply proven and standardized results of intestinal function restoration and consistent neuromodulation methodologies [46, 47].
Thus, various modifications of the intestine electrostimulation method showed high efficiency in terms of treatment and prevention of intestinal paresis in patients who had undergone abdominal surgery.
Conclusions
In this study, the patients admitted to the clinic had acute surgical pathologies. 30% of the patients were admitted with acute appendicitis, 25% with acute cholecystitis, 20% with acute intestinal obstruction, 15% with perforated gastric ulcers, 12% with duodenal ulcers, and 10% with a strangulated hernia of the abdomen. In all the patients, acute surgical pathologies were complicated by peritonitis. Based on the conducted research, it was shown that the method of determining the temperature of the mucous membrane and cheek skin made it possible to reliably predict the occurrence of postoperative intestinal paresis in surgical patients with peritonitis. Of the 20 examined, 15 (75%) were predicted to have a low probability of developing postoperative intestinal paresis, and 5 (25%) patients were predicted to have a high risk of developing intestinal paresis – these patients underwent intestinal intubation using a probe.
The proposed intestine electrostimulation method was used in patients with a high risk of developing postoperative intestinal paresis. It was shown that the developed gastrointestinal electrostimulation method contributed to the complete restoration of intestinal motility on the first day, after which the probe was removed in 80% of cases. In 20% of cases, this method allowed full normalization of intestinal motility on the second day, while on the first day there was a partial restoration of intestinal motility. In general, the proven gastrointestinal electrostimulation method has high therapeutic efficacy and allows restoration of intestinal motility in a short time after surgery, preventing the development of postoperative paresis.
Thus, the methods presented in this study have significant clinical value for abdominal surgery. The method of predicting the risk of postoperative intestinal paresis based on determining the temperature of the mucous membrane and cheek skin helps to make a timely decision about the need for intubation. The developed gastrointestinal electrostimulation technique is effective for preventing the risk of intestinal paresis in patients with both high and low probability of problems with intestinal motility. Therefore, the proposed methods are of great practical value because they reduce the number of postoperative complications requiring repeated laparotomy and contribute to the normalization of the functional state of the gastrointestinal tract of patients after abdominal surgery.









