Introduction
Cancer is a major public health problem in the modern world. Every year, new cases of cancer are diagnosed around the world [1]. Cancer cells are altered cells that have escaped the mechanisms that regulate natural growth. Under normal circumstances, the balance between cell regeneration and cell death is maintained and new cell production is adjusted so that the number of specific cell types remains constant [2]. But due to environmental or inherited genetic mutations, the mechanisms that control the normal growth of the cell are stopped, causing the cell clones to expand significantly, leading to the production of a tumour or neoplasm. The current strategies for treating cancer include chemotherapy, surgery, and radiation therapy. Chemotherapy is the main treatment of choice. But chemotherapy drugs are often associated with drug-induced damage to healthy cells and tissues, and do not specifically target cancer cells. Also, cancer cells often turn into chemotherapy-resistant cells due to various factors, such as increased expression of detoxifying enzymes and drug transporters, and increased ability to repair DNA defects in cell organizations that involve apoptosis. Despite advances in treatment and diagnosis, including improvements in surgery, radiation therapy, and chemotherapy, these treatments may lead to severe side effects or increased resistance and can have a long-term negative impact on human health [3, 4]. Therefore, there is a need to develop other treatment options to add to the available treatments, and there is an urgent need for specific and targeted treatment of cancer cells that can alone treat cancer or be used as an adjuvant to reduce therapeutic doses of common anticancer drugs [5]. Some experimental studies have reported the therapeutic potential of bacteriocin against different types of cancer cells [6]. Bacteriocins are cationic peptides synthesized by ribosomes that are secreted by almost all groups of bacteria. Some bacteriocins have shown selective toxicity to cancer cells compared to normal cells [6]. This makes them candidates for research and clinical trials.
Aim
Due to the high prevalence of colon cancer and its therapeutic problems, this study was performed on colicin E7 to evaluate its anti-colon cancer properties.
Material and methods
In silico analysis
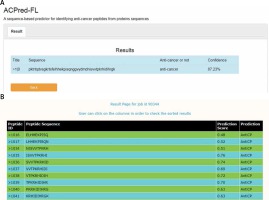
To determine the possibility of colicin E7 as an anticancer drug, it was evaluated in the ACPred-FL [7] and AntiCP 2.0 database [8].
PCR for identification of colicin E7
The PCR reaction was performed using specific primers for the presence of the colicin E7 gene. Then, the PCR product (colE7 gene) was electrophoresis on a 1.1% agarose gel at a voltage of 80 V for 70 min. Bacterial DNA purification was performed using the DNA purification kit (Korea, GeneAll) according to the instructions.
Plasmid vector
Pet32c expression vector was obtained from Merck, Germany. The pet32c vector was used to make a recombinant plasmid containing the colicin E7 gene. Before transferring the gene into the ligation vector, the pulsed vectors were first cut with the appropriate restriction enzymes.
In general, 1 µl of restricted enzyme BamHI FastDigest and HindIII FastDigest per 1 µg of plasmid DNA were used and incubated for 15 to 20 min at 37°C. They were inactivated at 80°C for 10 min. Ligation processes were done in the presence of vector and DNA insertion in a 3 : 1 molar ratio, T4 DNA Ligase Buffer, DNA ligase, and H2O. The total volume was 20 µl. The mixture was incubated at 22°C for 1 h, and then the enzyme was inactivated by heating at 65°C for 10 min. Then, according to the instructions of Sambrook and Russell, component cells of E. coli were prepared. After that, the transformation was done as follows.
The competent E. coli bl21 bacterial cells were placed on ice until they melted. Then, 8 µl of the ligation mixture was added to the micro tube containing 100 µl of the competent cell and incubated on ice for 30 min. Then, it was heated at 42°C for 50 s in a hot water bath. Then, 250 µl of SOS medium (yeast extract 5.0%, tryptone 2%, 5.2 mM glucose, 10 mM NaCl) was added to the microtube and incubated at 37°C for 1 h in a shaker incubator. Finally, 250 µl of the transformation reaction was transferred to a 0.1 mM Isopropyl β-D-1-plate, 100 g/ml ampicillin containing LB medium containing IPTG (thiogalactopyranoside) and 40 ml/µg Gal-X 40, and incubated overnight at 37°C.
Identification of recombinant E. coli
Recombinant colonies by white-blue colony on agar medium containing IPTG (1.0 mM) ampicillin 100 µl/ml and Gal-X 40 µg/ml were detected. Also, to confirm the cloning, plasmid extraction by Miniprep was performed. Then, positive recombinant colonies obtained from the previous screening under the influence of single and double restriction enzymes Hind III FastDigest and BamHI and the presence and size of the gene inserted in them was evaluated. The digested plasmid was examined by agarose gel electrophoresis.
Protein purification
Colicin E7 was purified using a Thermo Scientific HisPur Ni-NTA Spin Purification Kit. Also, the concentration and protein identification was performed by cell biolabsAKR-130 his-tag protein ELISA kit.
Effect of colicin E7 on HT29 cell line
Initially, cells in the logarithmic growth phase were trypsinised, implanted in 96-well plates, and incubated in a G: incubator. After 24 h, different concentrations of protein were added to the cell, and after the required time they were examined by MTT method. It should be noted that a concentration of 5 µM Oxaliplatin was used to treat HT-29 cells as a positive control.
qPCR for identification of the effect of colicin E7 on HT-29
Firstly, RNA was extracted using an RNA extraction kit (GENEALL, South Korea) and subjected to cDNA synthesis using a SCRIPT cDNA Synthesis kit (Germany). Secondly, the specific primers for the bcl2 and P53 genes were designed using GenScript software and synthesized. The ability of the primers for each specific gene to amplify the appropriate amplicon length was evaluated using 4 µl of cDNA in a total volume of 20 µl per reaction in a Mastercycler® RealPlex 2 (Eppendorf, Germany) with SYBR Green PCR Master Mix. The thermocycling conditions consisted of an initial denaturation for 15 min at 95°C, followed by 40 cycles at 95°C for 15 s, 60°C for 40 s, and 68°C for 20 s. The RT-qPCR results were then analysed quantitatively to estimate the levels of bcl2 and P53 mRNA.
Results
The potential of colicin E7 as an anticancer peptide
Our analysis demonstrated that colicin E7 has 87.23% confidence for anticancer peptide by ACPred-FL program and it was also confirmed as a potent anticancer peptide by AntiCP 2.0 (Figure 1). Both databases showed that colicin E7 has a potential to be an anticancer peptide.
Detection of colicin E7 in E. coli
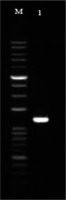
As described in the methods, the colicin primer was designed based on the colicin E7 gene sequence. The best result was found at an annealing temperature of 59.6°C. Colicin gene amplification was performed by PCR method and in order to confirm the amplification of the DNA, and a fragment of the desired PCR products was electrophoresis on 1% agarose gel, and then colicin E7 gene was purified from the agarose gel. A PCR reaction was performed using specific primers of the colicin E7 gene; it formed the 1728 bp fragment that belongs to this gene (Figure 2).
The purified colicin E7 gene was then inserted into the pet32c vector. In the next step, the ligation product was transferred to E. coli bl21, and then it was grown in LB agar medium containing 100 µg/ml ampicillin, 0.1 mmol/l IPTG, and 40 µg/ml X-Gal at 37°C for 24 h.
After overnight incubation at 37°C, positive recombinant colonies were selected on agar medium based on the blue/white screening method. White colonies were collected and cultured on new LB agar plates containing 100 µg/ml ampicillin, after which plasmid was extracted and enzymatic digestion was performed to confirm the presence of colicin E7 gene.
DNA sequencing was also performed to investigate the presence as well as the placement of the colicin E7 gene ligation in the recombinant vector. The results of colicin E7 gene blasting using the Chromas program showed that the homologation was 100%. After confirmation, recombinant pet32c-colicinE7 vectors were stored at 20°C until use. Then, colicin E7 was purified and the effect of it was evaluated against HT-29 cells. The results are shown in Figures 3 and 4.
Figure 3
Expression of p53 in HT-29 cells. Oxaliplatin was evaluated as a control. In the presence of colicin E7, the expression of p53 gene was increased
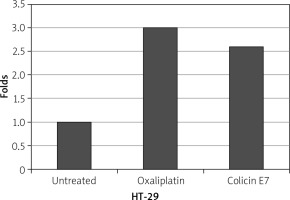
Figure 4
Evaluation of bcl-2 expression in HT-29 cells. Oxaliplatin as a control. In the presence of colicin E7, the expression of bcl-2 gene was decreased
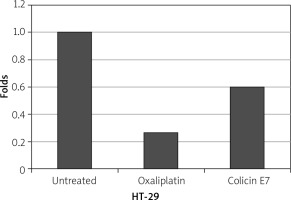
After exposure of HT-29 cells to colicin, the expression of p53 and bcl-2 were evaluated, which is shown in Figures 3, 4. The results showed a decrease in the expression of blc2 and an increase of P53 genes. The results of the expression of cancer genes show the effect of colicin E7 on HT-29 cancer cells. The qPCR method was used to evaluate the expression of the gene, which in turn indicates the expression of the protein.
Discussion
Over the past half century, cancer has become a serious and threatening human health problem. According to new data released by the World Health Organization (WHO), 2.8 million people have died from the disease and 14.1 million new cases of cancer worldwide have been reported [9]. Worldwide, colon cancer is the third most common type of cancer, accounting for 10% of all cancers. In 2012, there were 1.4 million new cases of the disease, as well as 694,000 deaths. The disease is more common in developed countries, and 65% of cases are found in these countries [10]. Some research shows that bacteriocins have anti-cancer activity against tumour cells. Research on colicins and their cytotoxic effects on human tumour cells began about 30 years ago, when the first reported colicin was colicin E3, which had significant cytotoxic effects on human HeLa cells [11].
In the present study, after exposure of HT-29 cells to colicin E7, expression of p53 and bcl-2 were evaluated, and the results showed a decrease in the expression of bcl2 and an increase of P53 genes. The results of the expression of cancer genes showed the effect of bacteriocin colicin on cancer cells HT-29. Oxaliplatin was also used for control. The expression of bcl-2 gene decreased in HT-29 cells, in the presence of colicin E7, which was near to the effect of oxaliplatin. Also, the expression of p53 in HT-29 cells in the presence of colicin E7 was effective. Although colicins, as a promising group of bacteriocins, appear to have anticancer activity against cancer cells, all aspects of their activity remain unknown [12].