Introduction
Acute pancreatitis (AP) is an inflammatory process of the pancreas, which is a life-threatening gastrointestinal disorder requiring hospitalization [1, 2]. Gallstones and alcohol have been attributed as the cause of more than 70% of all cases of AP [3]. As per revised Atlanta classification 2012, AP is classified into interstitial oedematous type or necrotising type, depending on the presence of pancreatic necrosis. Severe AP is defined as persistent organ failure > 48 h [4]. Acute interstitial pancreatitis occurs in 80% of cases of AP with favourable prognosis. However, acute necrotising pancreatitis is a serious form of AP with expected morbidity and mortality ranging between 30% and 50% [5]. Around half of the mortality is in the first week, the primary reason being the systemic inflammatory response. Sepsis-related complications, infective pancreatic necrosis, and multi-organ dysfunction are the usual causes of death in the later period [6, 7].
It is crucial to identify early and to triage severe AP patients to intensive care in order to prevent morbidity and mortality. The severity of AP can be stratified through serum markers, imaging methods, and complex scoring systems, to improve clinical judgement. The Acute Physiologic Assessment Chronic Health Evaluation II (APACHE II) score, Ranson score, the Bedside Index for Severity in Acute Pancreatitis (BISAP) score, and the Glasgow-Imrie criteria are the common scores used, but they are cumbersome and must be re-assessed at 48 h. Also, the CT severity index (CTSI) is used to assess the severity of AP, with the limitation that it has to be done 48–72 h after the onset of the acute event and cannot be used in acute renal failure. Moreover, none of these modalities can assess the severity of all patients accurately and in a timely manner to prioritise early referrals to specialised centres, intensive care, and treatment protocols to prevent complications related to severe inflammations, leading to sepsis and multi-organ dysfunction. Serum markers for predicting severity include C-reactive protein (CRP), serum procalcitonin, interleukin (IL) 6 (IL-6), IL-8, IL-12, IL-15, and IL-17, polymorphonuclear elastase, and trypsin activation peptide [8, 9]. Of these, CRP, IL-6, and procalcitonin are the most commonly used, despite none predicting the severity accurately. CRP and albumin (ALB) are positive and negative acute-phase proteins, respectively. CRP rises during inflammation, while albumin decreases. Hence, the CRP/ALB ratio can correlate positively with the degree of inflammation. This marker has been used as an indicator of prognostic factors for the prediction of overall survival for patients undergoing pancreatic surgery, prognostic score, and prognostic score in AP [10–13].
Aim
The present study investigated the CRP/albumin ratio for predicting the severity and mortality in AP patients.
Material and methods
Methodology
All consecutive patients with AP attending the gastroenterology unit of a tertiary care centre from March 2020 to April 2021 were prospectively enrolled in our study. AP was diagnosed by patients requiring 2 of the following 3 criteria: pain in the epigastrium, radiating or not to the back, elevation of amylase and lipase levels more than 3 times of upper limit of normal, and evidence of inflammation in cross-sectional imaging [14]. Those with age below 18 years, chronic pancreatitis, pancreatic carcinoma, patients with acquired immunodeficiency syndrome, and pregnancy were excluded from the study. Severity of AP was defined as per revised Atlanta classification 2012, as follows: If there is no organ failure or local complications, then it is mild AP; moderately severe AP is defined by organ failure persisting for less than 48 h or development of local complications; and persistence of organ failure for more than 48 h is defined as severe AP [4, 15]. During the study period, a total of 116 patients were diagnosed with AP. Blood samples for haematological and biochemical data were obtained within 1 h of admission. The APACHE II, CTSI, and BISAP score were also calculated upon admission. Serum ferritin was estimated on the day of admission, and the CRP/albumin ratio was calculated from the CRP and serum albumin report. All patients were followed up until death or up to 45 days after being discharged from the hospital. Written informed consent was obtained from all patients. The study was approved by the intuitional review board, and ethical clearance was taken for the study.
Alcohol as an aetiology of pancreatitis was considered when heavy alcohol intake or binge drinking was documented. Biliary pancreatitis was considered if there was evidence of gallstones or dilated common bile duct on ultrasonography and/or features of obstructive jaundice as suggested by altered liver biochemistry. Development of AP after endoscopic retrograde cholangiopancreatography (ERCP) was termed as post-ERCP pancreatitis. Hypertriglyceridaemia-induced pancreatitis was suggested if serum triglyceride value > 1000 mg/dl without any other cause. If no aetiology was found after excluding all possible causes, then idiopathic AP was considered.
Statistical analysis
All statistical analyses were performed using SPSS software, version 18.0 (SPSS Inc., Chicago, IL). Continuous variables are presented as mean and standard deviation. Categorical variables are presented as frequency and percentage. Continuous variables were compared using Student’s t-test, and categorical variables using the χ2 test. Univariate and multivariate analyses were done using the Cox regression method. Kaplan-Meir survival analysis of patients with AP by CRP/albumin ratio and ferritin levels was calculated. In addition, the predictive ability of each predictor was assessed by calculating the area under the curve (AUC) applying receiver-operating characteristic (ROC) curves for severity of AP. P-values less than 0.05 were considered statistically significant.
Results
Demographics and laboratory features of AP
The demographic profile of all AP patients, as shown in Table I, revealed that mean age was 40.63 ±5.49 years with a male predominance (73%) of AP. Alcohol was the most common aetiology (46.6%) followed by gallstones (39.8%), idiopathic (6%), post ERCP (5.1%), and hypertriglyceridaemia (3.5%). Mean CRP, albumin, and ferritin were 12.05 ±7.49 mg/l, 3.52 ± 0.79 mg/dl, and 919.13 ±735.72 ng/ml, respectively; the CRP/albumin ratio was 3.54 ±2.37. The mean CTSI, APACHE II, and BISAP scores at day 0 were 7.17 ±2.37, 0.69 ±0.78, and 5.56 ±4.72, respectively. In addition, the mean calcium and creatinine were 9.4 ±1.48 mg/dl and 1.6 ±1.4 mg/dl, respectively. Nineteen patients in our study had underdone intervention due to complicated fluid collections, 3 patients had undergone surgical necrosectomy, 10 patients had percutaneous catheter drainage, and 6 patients had endoscopic drainage, either a plastic stent or lumen apposing metal stent, depending on the nature of the fluid.
Table I
Baseline characteristics of patients with acute pancreatitis (n = 116)
[i] WBC – white blood cell count, AST – aspartate aminotransferase, ALT – alanine aminotransferase, ALP – alkaline phosphatase, CRP – C-reactive protein, CTSI – computed tomography severity index, ERCP – endoscopic retrograde cholangiopancreatography, APACHE – acute physiology and chronic health evaluation II, PCD – percutaneous catheter drainage.
Different parameters according to severity of AP
A comparison of different laboratory and prognostic markers according to the severity of pancreatitis is shown in Table II. In our study, 30 patients had severe AP, 47 has moderately severe AP, and 39 had mild AP, defined as per the Atlanta classification 2012 [4]. The ages of patients were similar in all severities of AP. The haematocrit was significantly higher in severe AP than in moderately severe and mild AP (p < 0.001). The mean ferritin and CRP/albumin ratio was also significantly higher in severe AP as compared to moderately severe AP and mild AP (p < 0.001). The mean CTSI, APACHE II, BISAP score, serum creatinine, and calcium were also significantly greater in severe AP as compared to moderately severe and mild AP. However, total white blood cell count (TWBC), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP), were similar in all grades of severity of AP.
Table II
Comparison of different parameters according to grade of acute pancreatitis
Mortality in AP and its predictors
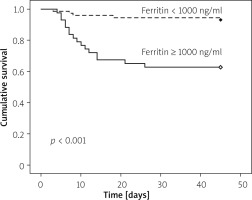
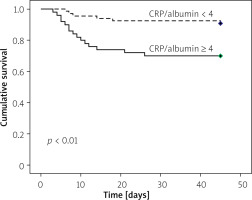
The predictors of mortality of AP patients in our study are shown in Table III. Out of 116 patients of AP, 21 patients died (18%): 4 patients with moderately severe AP and 17 patients with severe AP died in our study. In univariate Cox regression analysis, creatinine, CRP, Albumin, CRP/albumin, ferritin, CTSI, BISAP on day 0, and APACHE II were found to be predictors of mortality in AP. However, only the CRP/albumin ratio (AOR = 1.26, 95% CI: 1.02–1.56, p = 0.02) was found to be independent predictor of mortality in multivariate analysis. In addition, patients of AP with CRP/albumin ≥ 4 ng/ml had significantly lower cumulative survival as compared to CRP/albumin < 4 ng/ml in Kaplan Meir analysis (Figure 1). Similarly, patients of AP ferritin ≥ 1000 ng/dl had significant lower cumulative survival as compared to ferritin < 1000 ng/dl (Figure 2).
Table III
Factors predicting mortality in patients with acute pancreatitis, by Cox regression analysis
Figure 1
Kaplan-Meir survival analysis of patients with acute pancreatitis, by C-reactive protein (CRP)/albumin ratio
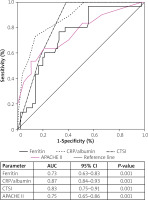
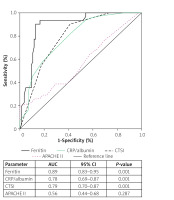
The accuracy of CRP/albumin, ferritin, CTSI, and APACHE II score for predicting the severity and development of necrotising pancreatitis was estimated by drawing an ROC curve (Figures 3 and 4). With regard to severity, CRP/albumin had the highest AUC (0.87, 0.84–0.93, p < 0.001) followed by CTSI (0.83, 0.75–0.91, p < 0.001), and it was higher than ferritin and APACHE II. However, ferritin had a higher AUC (0.89, 0.83–0.91, p < 0.001) than did CRP/albumin, CTSI, and APACHE II, suggesting that ferritin had a strong predictive value for the development of necrotising pancreatitis.
Discussion
In our study, we found that the mortality rate in patients with AP was 18%. The ferritin, CRP/albumin ratio, CTSI, and APACHE II values were significantly higher in severe AP. The CRP/albumin ratio was the only single independent predictor of mortality in patients with AP in multivariate Cox regression. However, patients of AP with CRP/albumin ≥ 4 and ferritin ≥ 1000 ng/dl had higher mortality than those with lower values of CRP/albumin and ferritin in Kaplan-Meir survival analysis. CRP/albumin had the highest AUC for predicting the severity of AP, and ferritin had the highest AUC for predicting the development of necrosis.
In our study, alcohol was most common aetiology of AP (in almost half of our patients). Indian studies have shown alcohol as the most common aetiology of AP, in contrast to the western population, probably due to higher alcohol abuse in the Indian population, mostly among males [16–20]. The mean age of AP patients in our study was around 40 years with a significant male predominance. Hence, AP is a disease of the young to middle-aged population, and the higher percentage of alcohol aetiology contributes to the male predominance of AP in our study. However, the incidence of idiopathic AP was much lower in our study, as compared to the higher value (36%) reported by a previous study from Kerala, India [21].
The mortality rate among AP patients in our study was 18%, which is higher than the average reported overall mortality rate for this disease. Our hospital is a tertiary care referral centre, and most of the AP patients admitted to our hospital had either moderately severe or severe AP, contributing to the higher mortality rate among AP patients. Hence, the type of disease pattern has a significant influence on mortality. Moreover, almost half of our study population had alcohol-induced AP, and a recent meta-analysis has shown that alcohol-induced AP has higher mortality than biliary and post-ERCP pancreatitis [22]. Other studies also reported a higher mortality rate of around 20% in their studies [13, 23].
Previous studies on AP revealed that the severity, as assessed by presence of organ failure, was a major determinant of mortality [15, 21]. In our study, the patients of severe AP had high levels of inflammatory markers like CRP and ferritin, suggesting early severe inflammation in this group of patients. Numerous studies evaluated the role of inflammatory cytokines IL-6 and IL-10, and found that they might be associated with clinical severity and fatal outcome [24, 25]. However, these cytokines are expensive and not available in many tertiary care centres; hence, cheap and widely available inflammatory markers like CRP, albumin, and ferritin might be useful in early triaging of severe AP patients for proper and intensive management.
In the present study, the CRP/albumin ratio was the only independent predictor of mortality in our AP patients, and a CRP/albumin ratio ≥ 4 was significantly associated with poor survival. Both high CRP and low albumin were associated with poor survival in patients with AP [26, 27]. Hence, the CRP/albumin ratio was advocated to predict the outcome in AP. Moreover, this ratio is not contemporary to AP, as it was previously used to prognosticate the patients of colorectal cancer, lung cancer, and sepsis [28–30]. In sepsis, the CRP/albumin ratio was found to be better than CRP alone in predicting mortality [31]. Because CRP and albumin are simple, cheap, and non-invasive markers that can be readily measured in most laboratories, the CRP/albumin ratio appears to be a promising marker to predict the outcome of AP early to guide further management.
In the current study, serum ferritin was not found to be a predictor of mortality. However, high ferritin value (≥ 1000 ng/ml) was associated with pancreatic necrosis in patients of AP in our study. Serum ferritin is an inflammatory biomarker that has been proven to have superior ability to predict outcomes in patients with end-stage liver disease and pancreatic cancer, and it has also been found to be elevated in COVID-19 infections [32–34]. Ferritin, one of the components of the COVID-19 scoring system, was also a factor predicting the mortality of COVID-19-infected patients admitted to ICUs [35]. Despite being a novel inflammatory biomarker in several inflammatory conditions, including COVID-19 infection and terminal cancer, the role of ferritin has not been evaluated in patients with AP until now; hence, ferritin might serve as a helpful tool to predict outcomes in patients with AP, because other predictive tools in AP such as APACHE II are very complex, requiring 18 parameters to calculate them. Therefore, large prospective studies are needed to evaluate the role of ferritin in patients with AP.
Our study has some limitations. This was a single-centre study with a small study population, and we only measured all parameters in the first 24 h of admission; no data are available on repeated measurements. Long-term follow-up data were not available in these patients. Despite this, we present a prospective study evaluating the role of a simple, cheap inflammatory biomarker to predict outcomes in AP patients, adding great strength to our study.
Conclusions
It is a great challenge for clinicians to predict organ failure and mortality in AP because no scoring system has proven to be effective in the guidance of early management. The CRP/albumin ratio is a cheap and simple biomarker, which has been found to be an independent predictor of mortality in patients with AP. In addition, serum ferritin played a predictive role in the development of pancreatic necrosis despite failing to prove its role in predicting mortality. Large prospective studies are needed to evaluate the role of the CRP/albumin ratio and ferritin in assessing the outcome of patients with AP.