Introduction
It is generally accepted that the gastrointestinal tract, especially the colon, is constantly exposed to reactive oxygen species (ROS) originating from endogenous and exogenous sources [1, 2]. ROS are radicals, ions, or molecules having a single unpaired electron in their outermost shell of electrons; therefore, they are characterised as highly reactive. ROS can be categorised into two groups: free oxygen radicals and non-radical ROS. Among free oxygen radicals the following should be mentioned: superoxide (O2•), hydroxyl radical (•OH), nitric oxide (NO•), organic radicals (R•), peroxyl radicals (ROO•), alkoxyl radicals (RO•), thiol radicals (RS•), sulphonyl radicals (ROS•), thiol peroxyl radicals (RSOO•), and disulphides (RSSR). Non-radical ROS include hydrogen peroxide (H2O2), singlet oxygen (1O2), ozone/trioxygen (O3), organic hydroperoxides (ROOH), hypochloride (HOCl), peroxynitrite (ONO-), nitrosoperoxycarbonate anion (O=NOOCO2-), nitrocarbonate anion (O2NOCO2), dinitrogen dioxide (N2O2), nitronium (NO2+), and highly reactive lipid-or carbohydrate-derived carbonyl compounds [3–6].
To keep a steady-state control over ROS production-detoxification and to prevent harmful effects, organisms have evolved defensive systems, e.g. scavenging enzymes [7]. The most significant antioxidant enzymes include superoxide dismutases (SODs) converting superoxide to less reactive H2O2, catalase (CAT) reducing H2O2 to water and molecular oxygen, and glutathione peroxidases (GPxs) that eliminate H2O2 by the use of reducing power derived from glutathione [8]. These enzymes are considered as the first-line defence antioxidants and are thought to be highly significant in the prevention of oxidative damage.
Aim
Therefore, in this work we try to assess immunohistochemical expression of CAT protein not only in adenocarcinoma patients but also in precancerous lesions including tubular, villous, and tubulovillous adenomas.
Material and methods
The group of patients
This study was performed on resected specimens obtained from 122 patients who had undergone surgical resection for primary sporadic colorectal cancer, and from 120 patients who had undergone colonoscopy at the Municipial Hospital in Jaworzno (Poland). All the specimens were obtained with the consent of the patients. In all cases, an experienced pathologist reviewed the haematoxylin and eosin (H + E) slides of the adenomas or primary tumours to confirm the pathological features.
The subject population of colorectal cancer patients comprised 60 men and 62 women. The tumours of the patients were classified histopathologically as adenocarcinoma according to the WHO grading system: grade 1 – 61 patients; grade 2 – 35 patients; and grade 3 – 26 patients.
The population of patients with adenoma comprised 49 men and 71 women. The adenomatous polyps were classified as tubular adenomas – 46 patients; villous adenomas – 37 patients; and tubule – villous adenomas 47 patients.
The design of the study was approved by the ethical committee of the Jerzy Kukuczka Academy of Physical Education in Katowice. The study was supported by grant KNW-1-043/N/5/0 of the Medical University of Silesia.
Immunohistochemistry of catalase protein
Paraffin-embedded, 4 µm-thick tissue sections were stained for rabbit polyclonal anti-CAT antibody obtained from GeneTex (cat. no. GTX110704). Deparaffinisation of all sections was performed through a series of xylene baths, and rehydration was performed through graded alcohol. To retrieve the antigenicity, tissue sections were treated three times with microwaves in a 10mM citrate buffer (pH 6.0) for 5 min each. Subsequently, antigen retrieval sections were incubated with rabbit polyclonal anti-CAT antibody (final dilution 1 : 600). The En-Vision method (Dako En-Vision Kit/Alkaline Phosphatase detection system) was used according to the manufacturer’s instructions. The bound primary antibody was detected using the New Fuchsin Substrate system (DAKO A/S).
Immunohistochemical analysis
We graded the immunoreactivity by using a semi-quantative approach. Immunohistochemical reaction for CAT was classified into four groups according to the intensity of immunohistochemical reaction: 0 – negative, 1 – weak, 2 – moderate, and 3 – strong. The intensity of immunohistochemical reaction in the inflammatory cells of lamina propria was described as strongly positive. Diffuse staining with the staining intensity weaker than that of inflammatory cells was characterised as moderately positive. Faint or focal staining was described as weakly positive. Heterogeneity was defined as the proportion of cancer cells showing a positive reaction to the total number of cancer cells and was graded from 0 to 3 by assessment: 0 demonstrated negative staining, 1 represented less than 10%, 2 represented 10–50%, and 3 represented more than 50% of cancer cells with positive reaction. The results of intensity of staining and heterogeneity were combined and scored as follows: 0 represented negative, 1 and 2 represented low, 3 and 4 represented moderate, and 5 and 6 represented high expression.
Results
In colorectal mucosa without any pathological changes CAT was predominantly localised in infiltrating mononuclear cells of lamina propria. Positive reaction was detected also in fibroblast-like cells, which were scattered around the crypts. In those cells immunoexpression of CAT was characterised as strong (Figure 1 A). Importantly, some fibroblast-like cells demonstrated also weak expression. In cells of the crypts the intensity of CAT expression was described as weak. Weak expression was detected also around blood vessels (Figure 1 A).
Figure 1
Immunoexpression of catalase (CAT) in colorectal adenomas and adenocarcinomas. A – CAT immunoexpression in cells of lamina propria (red arrows), fibroblast-like cells around the crypts (black arrows) and walls of blood vessels (arrowhead) in healthy colorectal tissue. B, C – CAT immunoexpression in samples of tubular adenomas with low-grade dysplasia. Positive reaction was detected in cells of lamina propria (red arrows) and fibroblast-like cells around the crypts (black arrows). CAT immunoreactivity was also demonstrated in apical parts of the crypts (arrowhead). D – CAT immunoexpression in tubule- villous adenomas with high grade of dysplasia was demonstrated in cells of lamina propria (red arrows) and apical parts of the crypts (arrowhead)
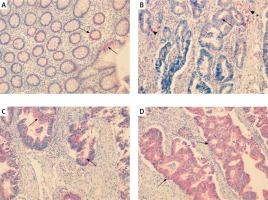
In adenoma samples strong immunoexpression was detected in infiltrating mononuclear cells within lamina propria. The scattered fibroblast-like cells localised mainly in the close vicinity of changed crypts showed positive strong immunoexpression as well. In neoplastic cells moderate staining appeared confined to the luminal side of the cytoplasm (Figures 1 B–D).
High expression of CAT was significantly associated with grade of dysplasia (high grade vs. low grade, p = 0.037) (Table I).
Table I
The correlation between CAT immunoexpression and clinicopathological variables in colorectal adenoma patients
In adenocarcinoma samples, expression of CAT was detected in cytoplasm of stromal and cancer cells. Expression in cancer cells was characterised as moderate or weak, whereas expression in stromal cells was described as strong.
High expression of CAT was significantly correlated with histological grade of tumour (G1 vs. G2 vs. G3, p = 0.003) and depth of invasion (T1 vs. T2 vs. T3 vs. T4, p = 0.033) (Table II).
Table II
The correlation between catalase immunoexpression and clinicopathological variables in colorectal adenocarcinoma patients
Discussion
The increased localised ROS in cancer cells needs to be buffered from reaching a level that incurs cellular damage [3]. An increasing body of evidence points to a prominent role of H2O2 as one of the most significant ROS in cancer pathogenesis. Recent data suggest that it may cross cellular membranes through specific members of the aquaporin family. In addition to the mitochondria, H2O2 might be generated also in peroxisomes. In these organelles, superoxide and H2O2 are generated through xanthine oxidase in the peroxisomal matrix and peroxisomal membranes [3–6]. H2O2 has been reported to participate in regulation of cell proliferation and induction of the transformed phenotype. For example, when human CAT was expressed in Nox1-expressing NIH 3T3 fibroblasts, those cells reverted to a normal appearance and tumour was no longer produced in athymic mice [9–16]. Targeted delivery of galactosylated CAT with either 3,5-di(ethylamino-2,2-bisphosphono) benzoic acid (Bip) or polyethylene glycol (PEG) were effective in inhibiting bone metastasis of tumour-bearing mice [17]. These findings suggest that CAT is the main antioxidant enzyme, which regulate the level of H2O2 and may contribute to cancer metastasis. Nevertheless, most malignancies have been shown to contain low levels of CAT, which may be due to the impairment of peroxisomal biogenesis in malignant cells. Given that peroxisomes are usually decreased in cancerous tissues, CAT might also be expressed in other parts of the cell, for example in the cytoplasm and mitochondria [18, 19]. In a study by Jaruga et al. CAT activity was lower in lung carcinoma when compared with normal tissue [20]. Coursin et al. investigated immunoreactivity of antioxidant enzymes in human lung carcinoma and found that CAT was negative in the neoplastic cells [21]. Kwei et al. demonstrated the same down-regulation of CAT expression in the squamous cell carcinomas generated using a mouse three-stage carcinogenesis model [22]. Down-regulated CAT expression has also been reported in ascites tumour cells, Morris hepatomas, Lewis lung carcinomas, and tumourigenic hamster kidney cells as compared to their respective normal cell types [13]. These observations are consistent with a study by Sun et al., who showed that immortalisation and transformation of mouse liver cells with SV40 (simian virus 40) resulted in a decrease in CAT expression contributing to oncogenesis by enhanced ROS production in transformed cells [23]. Finch et al. demonstrated that the loss of CAT expression is in part responsible for the enhanced malignant potential of the 6M90 cell line. In contrast, increased expression of CAT inhibited proliferation and tumour formation in the malignant 6M90 keratinocyte cell line at least in part through the EGF-R pathway. Interestingly, the parental line with CAT activity of 7 Amol/min/mg protein was tumourigenic while the cell line MTOC2 with the CAT transgene and CAT activity of 40 Amol/min/mg were characterised by low tumourigenicity [24].
In our study we used a large set of colorectal adenomas and adenocarcinomas to assess the expression of CAT at the protein level by the use of immunohistochemistry. To our knowledge, no previous study has assessed the clinical significance of CAT protein expression in colorectal neoplasia. Both in adenomas and adenocarcinomas CAT immunoexpression has been detected mainly in infiltrating mononuclear cells of lamina propria or cancer stoma. Nevertheless, expression of CAT has also been observed in cancerous cells, especially in apical parts of the cells. In comparison to controls, in adenoma samples the expression of CAT protein was upregulated. In adenomas with a high degree of dysplasia most samples showed a moderate level of CAT protein expression (about 39% of patients). In contrast, in adenomas with low degree of dysplasia 34% of samples revealed high level of CAT expression, and interestingly 30% demonstrated low level of immunoreactions. In comparison to adenoma samples, in cancer tissues, expression of CAT protein was decreased. Also in those samples, positive reaction was detected mainly in cells of cancer stroma. However, weak expression of CAT has also been demonstrated in cancerous cells. Interestingly, we did not observe any correlation between expression status and type of adenoma. We mention this because it is generally accepted that villous adenoma possess the highest capacity to transform into cancer.
In cancer patients CAT immunoexpression was significantly correlated with histological grade of tumour and depth of tumour invasion. Strong reaction was a characteristic feature of G1 tumours whereas weak immunoexpression was described mainly in G3 tumours. Furthermore, as mentioned above, the depth of tumour invasion was also a significant feature. T1 tumours were mainly characterised by strong immunoexpression of CAT, in contrast to T3 and T4, in which cells were mainly described by weak expression.
The results of our study concur with the study by Skrzydlewska et al. These researches revealed the highest increase in activity of Cu, Zn-SOD, HSH-Px, and GSSRG-R as well as decrease in CAT activity (p < 0.001) in G3-grade adenocarcinoma and mucinous adenocarcinoma as well as in clinical IV stage of colorectal cancer [25, 26]. In contrast, expression of another antioxidant enzyme MnSOD in colorectal cancer samples was increased [27]. It seems that decreased activity of CAT in cancerous cells leads to accumulation of H2O2, which is responsible for damage of DNA and cell death. Therefore, the combination of increased expression of MnSOD and decreased expression of CAT in colorectal cancer cells may result in enhanced production of H2O2 and decreased detoxification of this compound. Probably the high level of MnSOD and decreased activity of CAT may create an antiapoptotic environment, which is especially susceptible to high frequency of mutations [27, 28].
The reason for decreased expression of CAT in neoplastic cells remains puzzling, but it seems that prolonged exposure to ROS is responsible for downregulation of CAT expression by hypermethylation of CAT promoter [28, 29]. Additionally, in such regulation of CAT expression, transcription factors seem also to be involved. The Protein Kinase B (PKB/Akt)/Forkhead Box O (FoxO) transcription factors pathway is probably the best-known regulator of CAT expression [30, 31]. Indeed, FoxO3a has been reported to bind to the rat CAT promoter and in addition the transactivating activity of this transcription factor is negatively regulated by the serine/threonine kinase Akt [31]. Interestingly, it has been demonstrated that CAT expression in human MCF-7 breast cancer cells is repressed by the PI3K/Akt/mTOR signalling pathway while FoxO3 seems not to play a significant role in this gene regulation [32]. Limited information is connected with the mechanism of CAT promoter regulation including the regulatory mechanism involved in CAT expression in cancer cells. Transcription factors, such as Nuclear Factor Y (NFY), Specificity protein 1 (Sp1), Peroxisome proliferator-activated receptor gamma (PPARγ), Forkhead box protein M1 (FoxM1), and POU domain class 2 transcription factor 1 (POU2F1/Oct-1), have recently been reported to bind the human CAT promoter and probably regulate transcription of CAT gene [33]. Glorieux et al. showed that the AP-1 family member JunB and retinoic acid receptor alpha (RARα) mediate CAT transcriptional activation and repression, respectively, by controlling chromatin remodelling through a histone deacetylases-dependent mechanism. This regulatory mechanism plays a critical role in redox adaptation to chronic exposure to H2O2 in breast cancer cells [33]. It must be noted that in some cancers, expression of CAT has been upregulated. In mesothelioma patients and in rat glioma cells the CAT protein level was increased conferring cellular protection against epirubicin and ionising radiation (137Cs γ-rays) respectively [34, 35]. Increased CAT expression has also been observed in tumours from patients with gastric carcinoma, skin cancer, and chronic myeloid leukaemia and in human HL-60 cancer cells rendered resistant to chronic exposure to H2O2 [36–38]. The high level of CAT expression was also a characteristic feature of several human cancer cell lines including gastric, oral, pancreatic, bladder cancer cells exposed to cisplatin, ascorbic acid, bleomycin, gemcitabine, mitomycin C, hormonal therapy, and ionising radiation [13]. Gupta et al. showed that high levels of ROS and low levels of catalase may increase cancer progression, which suggests that catalase may function as a tumour suppressor [39]. Low catalase expression in tumours compared to non-tumour tissues could serve as a valuable predictor of poor survival in patients with advanced HCC, and enhancement of catalase expression in tumours could be a useful therapeutic strategy for the treatment of HCC patients [40].
This study concerns only histochemical determination without any functional attempt. The direct measurement of reactive oxygen species (ROS) mentioned in the text in the samples collected from surgery and colonoscopy was not performed.









