Acute pancreatitis (AP) is a leading gastrointestinal reason for admission and readmission to the hospital. The global incidence of AP is steadily increasing. The disease has numerous risk factors; however, cholelithiasis and alcohol abuse are two of the most common causes, responsible for over 80% of cases [1, 2]. In the absence of alcohol or gallstones, detecting the possible etiology needs careful evaluation. The precise identification of AP etiology may contribute to the diagnosis of other severe disorders. While abdominal imaging is not always performed in the AP diagnostic process, it may occasionally reveal unexpected findings such as a tumor. Malignancies, such as pancreatic adenocarcinoma (PDAC), may present as AP, and data indicate that about 10% of subjects with PDAC present with AP [3]. Other malignancies, such as lymphoma, represent very rare AP etiological factors [4]. We present a case of AP caused by germinal center B-cell-like diffuse large B-cell lymphoma (GCB-DLBCL) with extranodal disease secondarily involving the pancreas.
A 38-year-old man without chronic diseases was admitted to the Department of Digestive Tract Diseases in Norbert Barlicki Memorial University Hospital with severe epigastric pain radiating to the back lasting for the last 10 days, with significant exacerbation of the symptoms in the last few days before hospitalization. The patient had a history of a holiday in Greece where those symptoms occurred. In addition, the man reported having consumed large quantities of alcohol during the holiday, but he denied regular alcohol abuse. There was no history of any recent trauma or use of medications and drugs. Blood tests showed mild microcytic anemia with the level of hemoglobin of 11.6 g/dl (RR: 13.5–18.0 g/dl), C-reactive protein of 17 mg/l (RR: 0.0–5.0 mg/l), a slightly elevated level of alkaline phosphatase of 127 units per liter (U/l) (RR: 30–120 U/l) with a normal level of γ-glutamyltransferase and bilirubin, a slightly elevated level of aspartate aminotransferase of 42 U/l (RR: 0–40 U/l) with normal alanine aminotransferase of 36 U/l (RR: 0–40 U/l), considerably elevated serum lipase of 2274 U/l (RR: 0–67 U/l), marginal serum albumin of 34.3 g/l (RR: 35–52 g/l), total protein of 53 g/l (RR: 60–80 g/l), slightly elevated CA19-9: 40.9 U/ml (RR: < 35 U/ml), normal glucose and creatinine, and no alcohol. In addition, no HIV, HBV, or HCV infection was detected in tests. Abdominal ultrasound was negative for cholelithiasis or biliary duct dilatation, but revealed a diffuse heterogenous, hypoechogenic image of the pancreas with no focal changes and with the main pancreatic duct dilated to 4 mm. In addition, a hypoechogenic, oval structure located in the mesogastrium with many adjacent mesenteric lymph nodes was observed. Due to the very strong abdominal pain with unsatisfactory response to analgesics and inconclusive abdominal ultrasound, computed tomography (CT) of the abdomen and pelvis was performed. It showed numerous abnormal retroperitoneal lymph nodes, an irregular thickened wall of the duodenum and blurred outlines of the pancreas with a separated area in the head and dilated main pancreatic duct (Figure 1). Mild AP was diagnosed, and analgesic treatment with fluid therapy was initiated. He had no evidence of organ failure or shock. Upper gastrointestinal endoscopy was performed, and antral and duodenal erythema was revealed, while a urease test was negative. There were no metaplasia, dysplasia or cancer cells in a biopsy specimen from the gastric antrum and body. After 6 days, significant clinical improvement was achieved, and the patient was discharged. Further diagnostics, including endoscopic ultrasonography (EUS), were planned in less than a month. Unfortunately, after 7 days, the patient was again admitted to the same department with jaundice and epigastric pain, which were not accompanied by fever or shivers. A blood test revealed an elevated level of alkaline phosphatase of 321 U/l (RR: 30–120 U/l, total bilirubin of 5.09 mg/dL (RR: 0.3–1.2 mg/dl), direct bilirubin of 2.88 mg/dl (RR: 0.0–0.3 mg/dl), aspartate aminotransferase of 300 U/l (RR: 0–40 U/l), and alanine aminotransferase of 577 U/l (RR: 0–40 U/l). An abdominal ultrasound scan was positive for dilatation of biliary ducts, both intra- and extrahepatic, and thickening of the duodenum wall. During hospitalization, an attempt of endoscopic retrograde cholangiopancreatography (ERCP) was made, but was ineffective due to diffuse, pathological infiltration of the descending duodenum, preventing further duodenoscope passage (Figure 2). Multiple biopsies from the infiltration were taken. Additionally, EUS with biopsy of the hypoechogenic, blurred head of the pancreas was conducted. During hospitalization, the levels of total and direct bilirubin were steadily raising. Both histopathological examinations confirmed the diagnosis of duodenal GCB-DLBCL with double expression of Bcl2/c-Myc, a high proliferation index of Ki-67 (90–100%), and immunophenotype of Bcl2+, Bcl6+, c-Myc+, CD10+, CD138-, CD20+, CD23-, CD3-, CD5-, CD56-, chromogranin-, CKAE1/AE3-, cyclin D1-, MUM1 protein-, PAX-5+, synaptophysin-. The patient was found eligible for hospitalization in the Department of General Hematology. Positron emission tomography/computed tomography (PET-CT) revealed metabolically active infiltration of the duodenum with numerous stimulated mesenteric lymph nodes. Despite the high levels of total (12.0 mg/dl) and direct bilirubin (11.0 mg/dl), rescue immunochemotherapy with rituximab, cyclophosphamide, vincristine, and prednisone (RCOP) was administered. After 5 days (1 day after the last dose of prednisone), significant reductions of total (5.26 mg/dl) and direct bilirubin (4.19 mg/dl), as well as a general improvement, were noted. After the third cycle of therapy, PET-CT demonstrated total metabolic and morphological regression of previously described duodenal and mesenteric infiltration. Currently, the patient is well, awaiting the next course of the therapy.
Figure 1
Abdominal computed tomography scan showing thickened wall of duodenum, indicated with red arrows
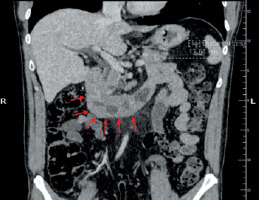
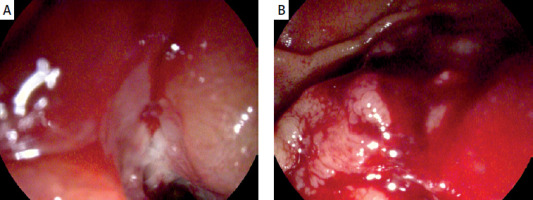
Figure 2
Pathological contact bleeding infiltration of descending part of duodenum preventing endoscopic drainage. Images from endoscopic retrograde cholangiopancreatography
The incidence of AP is expected to rise due to the rising occurrence of its risk factors. However, AP in the course of non-Hodgkin lymphoma is rare. Fewer than 1.4% of all lymphomas and 5% of all gastrointestinal tumors represent primary lymphomas of the gastrointestinal tract. DLBCL, a subtype of non-Hodgkin lymphoma, is characterized by aggressive malignancy, and its curability rate is estimated to be 60–70% [5]. On the other hand, non-Hodgkin lymphomas were found to be rare causes of obstructive jaundice symptoms, as well as exceptionally of AP [5]. The literature review revealed single cases of AP stimulated by lymphoma diagnosed de novo [6, 7]. Generally, only 0.2–2% of patients with lymphoma have pancreatic involvement at the time of diagnosis [6]. So far, AP induced by lymphoma has been described mainly in the pediatric population, HIV-positive status subjects, or patients treated with immunosuppressive drugs due to autoimmune diseases [6, 8–10]. None of these factors were confirmed in the present patient. In addition, he did not suffer from classic symptoms of lymphoma, such as weight loss, fever, and night sweats. Moreover, the patient’s medical history did not indicate typical risk factors of lymphoma, such as obesity, Helicobacter pylori infection, viral infection, exposure to toxic compounds, chemotherapy, or radiotherapy in the past. Our case report confirms the recommendation to pursue further examination in patients with AP without a clear etiology. In CT during the first hospitalization, the image suggested inflammatory changes secondary to AP. Nevertheless, further diagnostics, including EUS, were planned. The development of lymphoma was rapid, and the symptoms were severe, requiring a second hospitalization. The development of obstructive jaundice with duodenal stenosis preventing successful ERCP caused serious management challenges. Typically, the standard chemotherapy regimen for DLBCL includes rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP); however, these drugs require liver metabolism and biliary excretion [4]. Nevertheless, it was decided to use RCOP.
Our case report of AP leading to his diagnosis of DLBCL had unusual aspects from the presentation to the diagnosis. This case highlights the need for a broad differential diagnosis when the etiology of AP remains inconclusive. It is worth emphasizing that cancer should be considered as a potential cause of AP in the case of uncertain etiology of the disease, and lymphoma of the duodenum or pancreas should be listed as one of the potential risk factors of AP. In this case, the symptoms of AP and jaundice enabled an early diagnosis of DLBCL. In conclusion, AP may be a rare presentation of primary duodenal lymphoma, and adequate management can lead to proper diagnosis and a successful outcome, as seen in our case.










