Introduction
In the last 20 years, dramatic advances have been made in laparoscopic surgery, based on its minimally invasive nature, as a replacement for conventional laparotomy procedures in many fields of surgery, including gastrointestinal surgery [1, 2]. The impact of these advances has extended to hepatic resection procedures, and now many hepatic resections are performed laparoscopically [3].
We previously reported significantly favourable outcomes of laparoscopic partial hepatectomy compared to open partial hepatectomy, including reduced blood loss during surgery, reduced rate of superficial surgical site infection (SSI), and reduced postoperative inflammatory reaction [1, 4]. These results are achieved due to advances in the equipment used in laparoscopic surgery, and the improvement and standardisation of surgical procedures for laparoscopic hepatectomy. Although there are various advantages to laparoscopic surgery, including the magnifying effect, haemostatic effect of abdominal air pressure, and the minimal invasiveness of this procedure, complete laparoscopic minor hepatectomy (LH) is very difficult to perform in patients with liver cirrhosis because of the haemorrhagic liver and fibrosis of the liver parenchyma [5].
Aim
In this study, we investigated the postoperative outcomes in a series of complete LHs performed in patients with advanced cirrhosis, as well as the safety and efficacy of this procedure.
Material and methods
Patient population and selection
Laparoscopic hepatic resection was introduced in our hospital in 1998, and we gradually standardised the surgical procedure. Because a significant number of cases of laparoscopic hepatic resection had already accumulated by 2010, the procedures of laparoscopic hepatic resection were established. This study included patients who underwent this operation after 2010, when standardisation of the surgical procedure was established. A tumour size of < 10 cm was the main criterion that indicated LH; tumour number or tumour location was not considered as a criterion for indicating LH. However, not more than five sites of hepatic resection were considered as an indication for LH. Patients with main bile ductal involvement and/or metastasis to adjacent organs were not considered for LH. Moreover, LH was not considered when any complication occurred after other surgical procedures.
Between January 6, 2010 and December 21, 2018 we conducted liver resection for liver tumours in 837 consecutive patients in Osaka Medical College Hospital, Takatsuki City, Japan. Minor hepatic resection was performed in 112 of these patients for hepatocellular carcinoma (HCC), who had a pathological diagnosis of F4 cirrhosis [6]. LH for HCC with cirrhosis was performed for 71 patients, and open minor hepatectomy (OH) was performed for 41 patients. These 112 patients underwent hepatectomy with no other concomitant surgical procedure (i.e. colorectal). All patients were fully informed of the study design and provided their written informed consent to participate. This study was approved by the Ethics Committee on Clinical Investigation of Osaka Medical College Hospital (approval numbers 1828 and 1994).
We evaluated hepatic function using the Child-Pugh classification [7] of liver dysfunction. Criteria to convert laparoscopic to open hepatic resection were as follows: (1) when the liver stumps of both preserved and resected sides could not be expanded adequately, (2) when intraoperative bleeding could not be controlled, (3) when blood loss exceeded 500 ml, (4) when the total time of the Pringle manoeuvre (hepatic blood flow occlusion) exceeded 120 min, and (5) when intraoperative bile leakage indicated during the operation could not be improved. The patients who required conversion from LH to OH were analysed as part of the LH group.
Surgical procedure
In this series, all patients received potentially curative hepatic resection with the complete removal of the gross tumour with negative macroscopic margins. All procedures were performed by three experienced hepatobiliary surgeons (YI, FH, and KU) during the study period.
All procedures were performed with patients under general anaesthesia. The detailed open and laparoscopic surgical techniques routinely used in our department have been described in previous reports [4, 8–11]. Briefly, standard diagnostic and staging laparotomy was conducted. The liver was mobilised, and intraoperative ultrasonography (Prosound α7, Hitachi Aloka Medical Ltd., Tokyo, Japan) was routinely performed. Central venous pressure (CVP) was maintained at 0–3 mm Hg during parenchymal transection. Parenchymal transection was achieved using a surgical tissue management system (Thunderbeat, Olympus Inc., Tokyo, Japan) and a Sonop 5000 ultrasonic dissector (Hitachi Aloka Medical, Ltd.). Small vessels were ligated or coagulated using a soft-coagulation system. Intraparenchymal control of major vessels was obtained with non-absorbable sutures, whereas biliary and vascular radicle division was accomplished with stapling devices or non-absorbable sutures. The hepatic pedicle was always isolated to enable performance of the Pringle manoeuvre when needed. Intermittent clamping was applied, with 15-minute clamping and 5-minute release periods. During the resection procedure, the surgical margin was carefully confirmed using intraoperative ultrasonography to obtain a surgical margin of 5–10 mm when possible.
Data collection
Data examined included preoperative factors, surgical factors, and pathological factors. Preoperative factors investigated were age, sex, American Society of Anaesthesiology (ASA) classification, body mass index (BMI), viral infection status, presence of diabetes mellitus, total bilirubin level, albumin level, prothrombin time (PT), platelet count, aspartate aminotransferase (AST) level, alanine aminotransferase (ALT) level, indocyanine green retention rate at 15 min (ICG-R15), Child-Pugh classification, and prognostic nutritional index. Surgical factors included the conversion rate, surgical duration, intraoperative blood loss, and blood transfusion requirements. Pathological factors included the size of the largest tumour, number of tumours, and surgical margin status. “R” classification denoted the absence or presence of a residual tumour after surgery [12]. R0 resection refers to excision of the tumour in one piece without violating the tumour plane or achieving negative margins after sequential re-excision of the involved margins. R1 resection involves a microscopically positive margin anywhere, and R2 resection involves one or more macroscopically positive margin(s) with visible tumour.
Postoperative evaluation
The following parameters were evaluated: white blood cell (WBC) count, C-reactive protein (CRP) level, AST level, ALT level, platelet count, albumin level, total bilirubin level, PT, transfusion rate, pathological margins, postoperative complications, 30-day mortality, and hospital stay. Morbidity was graded according to Clavien-Dindo’s classification [13, 14]. SSIs were defined according to the Centre for Disease Control’s National Nosocomial Infection Surveillance system [15].
Definitions
Operative procedures were classified according to conventional terminology derived from the eight segments of the liver as per the Couinaud classification [16]. Anatomical resection was defined as resection of the neoplasm together with the portal vein related to the neoplasm and corresponding hepatic territory. Non-anatomical resection was defined as the resection of a lesion without regard to the segmental, sectional, or lobar anatomy.
Postoperative bile leakage and posthepatectomy liver failure were defined based on the criteria of the International Study Group of Liver Surgery [17, 18]. We defined massive ascites as ascites that could not be mobilised or as early recurrence that could not be satisfactorily prevented by medical therapy [19].
Statistical analysis
To minimise the effect of potential confounders on selection bias, propensity scores were generated using binary logistic regression analysis, which included the following variables: age, sex, ASA classification, BMI, hepatitis viral infection, diabetes mellitus, total bilirubin level, albumin level, PT, platelet count, AST level, ALT level, ICG-R15, Child-Pugh classification, number of tumours, largest tumour size, and tumour location. The choice of these variables was based on results of the univariate analysis and/or the known effect of specific factors on the selection of the type of intervention. Independent variables entered into the propensity model included the patients’ preoperative information. One-to-one matching between groups was accomplished using the nearest neighbour matching method, which was performed without replacement and using a calliper width of 0.2 standard deviations of the logit of the estimated propensity score. After propensity score matching (PSM), the two matched groups were handled as unpaired independent groups. Continuous variables are expressed as median±standard deviation. Results of univariate analysis were compared using Student’s t and χ2 tests, Mann-Whitney’s U test, Wilcoxon’s signed-rank test, or Fisher’s exact test, as appropriate. Factors that were found to be significant in the univariate analysis were included in multivariate logistic regression analysis to determine the adjusted odds ratios. Values of p < 0.05 were considered significant. All statistical analyses were performed using JMP version 12 (SAS Institute, Inc., Cary, NC, USA).
Results
In the LH group, the laparoscopic procedure was successfully completed in 66 patients. However, 10 patients (12.7%) were converted to OH because of bleeding from a hepatic vein branch, adhesion, and intraoperative bile leakage that could not be controlled laparoscopically and because the Pringle manoeuvre time exceeded 120 min; they were included in the OH group. By PSM, 28 of 71 patients in the LH group could be matched with 28 of 41 patients in the OH group. The baseline characteristics of the matched study population (56 patients) are summarised in Table I. There were no significant differences in the demographic or operative characteristics between the groups.
Table I
Patient demographic data
[i] ASA – American Society of Anaesthesiology, NA – not applicable, PSM – propensity score matching, LH – laparoscopic hepatectomy, OH – open hepatectomy, AST – aspartate aminotransferase, ALT – alanine aminotransferase, ICGR-15 – Indocyanine green retention rate at 15 min, PNI – prognostic nutritional index.
Surgical outcomes are presented in Table II. After PSM, in cases in which hepatic resection was laparoscopic, the Pringle manoeuvre was performed in 17 of 28 (60.7%) patients, and in open resections, the Pringle manoeuvre was performed in 11 of 28 (39.3%) patients (p = 0.106). The estimated blood loss was significantly lower in the LH group (25 ml; range: 0–450 ml) than in the OH group (310 ml; range: 0–1940 ml) (p < 0.001). There was no significant difference between the two groups regarding the operative time (p = 0.091), although the OH group tended to have a longer operative time than the LH group.
Table II
Surgical procedures and results
Early-stage complications following surgical treatment including the incidences of SSIs and remote site infections within 30 days postoperatively were compared. The incidence of superficial incisional, deep incisional, and space/organ SSIs was not different between the two groups (p = 0.313, 1.000, and 0.160, respectively). The LH group had a complication rate of 3.6% for Clavien-Dindo grade IIIa or higher, whereas the OH group had a complication rate of 35.7% (p = 0.003). Moreover, the incidences of refractory ascites and respiratory complications including pleural effusion were significantly different between the two groups (p = 0.019, p = 0.005, and p = 0.038, respectively). Overall, 8 (7.1%) patients had in-hospital mortality: posthepatectomy liver failure (PHLF) in 7 patients and postoperative bile leakage in 1 patient. After PSM, the LH group had no mortality, whereas the OH group had a mortality rate of 10.7% (p = 0.038).
The postoperative medical treatment was similar for the two groups, including intravenous electrolyte and balanced fluid solutions. Oral intake of fluid started on postoperative day 2. The median postoperative duration of intravenous medicine was 5 days in both groups. The postoperative length of stay was significantly longer in the OH group (14 days; range: 9–71 days) than that in the LH group (9 days; range: 5–65 days; p = 0.002).
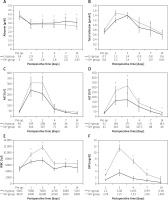
Postoperative AST levels peaked on day 1 and were almost normalised on day 7. Postoperative serum albumin levels, WBC counts, CRP levels, ALT levels, PTs, and platelet counts peaked on day 2 and then gradually normalised. Postoperatively, serum albumin levels, AST levels, ALT levels, WBC counts, and CRP levels, especially on the peak day, were significantly better in the LH group than in the OH group (p = 0.015, p = 0.010, p = 0.033, p = 0.003, and 0.003, respectively; Figures 1 A–F).
Figure 1
Postoperative changes in laboratory data. Postoperative serum albumin levels (A), total bilirubin levels (B), AST levels (C), ALT levels (D), WBC count (E), and CRP levels (F) in patients after hepatectomy. Postoperative serum albumin levels, AST levels, ALT levels, WBC counts, and CRP levels, especially on the peak day, are significantly better in the LH group than in the OH group (p = 0.015, p = 0.010, 0.033, p = 0.003, and p = 0.003, respectively)
*P < 0.05. AST – aspartate aminotransferase, ALT – alanine aminotransferase, WBC – white blood cell, CRP – C-reactive protein, LH – laparoscopic hepatectomy, OH – open hepatectomy.
The 1-, 2-, 3-, and 5-year OS rates were 94.6%, 91.7%, 79.5%, and 63.6%, respectively, with a median survival time of 27 months. The 1-, 2-, 3-, and 5-year recurrence-free survival (RFS) rates were 74.5%, 48.9%, 35.2%, and 17.6%, respectively. The 1-, 2-, 3-, and 5-year OS rates in the LH and OH groups were 100.0%, 93.3%, 81.7%, and 81.7% and 89.3%, 89.3%, 77.6%, and 51.7%, respectively (p = 0.260). The 1-, 2-, 3-, and 5-year RFS rates in the LH and OH group were 67.3%, 45.6%, 28.5%, and 28.5% and 82.2%, 53.3%, 41.5%, and 0%, respectively (p = 0.381).
Discussion
Factors suggested to affect the difficulty and invasiveness of hepatectomy include the operative time, blood loss, perioperative blood tests, and complications. In HCC complicated by cirrhosis, time is needed to expand the visual field because of the difficulty in mobilising the fibrotic liver, and dissection of the liver parenchyma does not always go as planned because of the hardness of the liver due to fibrosis. Many patients also have increased blood loss because of the difficulty in controlling blood loss during the surgery as well as decreased coagulability due to cirrhosis. The skill of the operator is also a factor. An increased incidence of PHLF is associated with increased perioperative blood loss, so it is essential to keep blood loss to a minimum. The incidence of PHLF in HCC with cirrhosis and postoperative complications consisting mainly of refractory ascites have tended to decrease in recent years, but preventing these conditions remains an important issue. Criteria for the indication of hepatic resection for HCC with cirrhosis have been investigated in numerous facilities to prevent early stage liver failure after liver resection.
Some recent reports [20–28] have confirmed the technical feasibility and safety of the laparoscopic technique for patients with liver tumours, but an ideal prospective, randomised study comparing laparoscopic and open hepatic resection has not yet been performed. This study retrospectively compared the degree of difficulty and invasiveness of laparoscopic and conventional open hepatic resections for liver tumours from the perspective of short-term outcomes in a single institution.
In our study, significantly better outcomes were found in the LH group, in terms of blood loss, postoperative complications, operative mortality rate, and postoperative length of stay. In terms of operative time, the laparoscopic group tended to have shorter operative times, but this was not significantly different between the groups. In the LH group, abdominal closure was not very time-consuming, whereas in the OH group, the skin incision was usually large, so abdominal closure often took an hour or more. A significant difference was also found in expansion of the visual field. Particularly for tumours located in segment 6, 7, or 8, the right lobe of the liver must be fully mobilised to secure a visual field when dissecting the liver parenchyma. However, in cases with comorbid liver cirrhosis, a liver enclosed by the ribs has poor mobility, which often makes mobilisation difficult. Therefore, mobilisation was time consuming in the OH group, whereas in the laparoscopic group the liver could be approached from the lateral side by changing the laparoscopic insertion port, enabling the view required for dissection of the liver parenchyma to be secured with minimal mobilisation [8]. Thus, the difference in the operative times may be a reflection of these factors.
In terms of blood loss, perioperative blood loss during hepatectomy includes blood loss from the hepatic arteries, veins, and portal vein. Interrupting the inflow blood during dissection of the liver parenchyma, which is the main cause of perioperative blood loss, is one way of reducing the amount of perioperative blood loss [29]. In both groups, the inflow of blood was interrupted as much as possible, implementing what is known as the Pringle manoeuvre [30], thereby inhibiting arterial and portal vein haemorrhage. However, LH is more advantageous than OH in terms of venous haemorrhage. In the LH group, venous haemorrhage was inhibited by increasing abdominal air pressure, but there was no corresponding method available in the OH group [31]. This factor is thought to be the reason for the differences in our study’s results. However, increasing intraabdominal pressure with pneumoperitoneum in patients with cardiac comorbidities during LH can reduce venous return, increase CVP because of reduced cardiac output, and lead to increased peripheral vascular resistance, which may conversely increase the risk of complications, so caution is needed [32, 33].
Lastly, in terms of perioperative blood test results and complications, although the laparoscopic group had no difference in the incidence of PHLF-related complications, the incidence of refractory ascites and respiratory complications including pleural effusion, and the operative mortality rate, were significantly lower. As reported previously, LH is a less invasive procedure than OH [34], and this fact is particularly notable in patients with comorbid cirrhosis, who have insufficient hepatic reserve. This is demonstrated by the fact that in perioperative blood test results, changes in short-term postoperative albumin levels were significantly lower in the OH group, whereas liver deviation enzymes, such as AST and ALT, and indicators of inflammatory responses, such as WBC and CRP, were significantly higher, indicating the significant difference in the invasiveness of the procedure.
LH was found to have various good outcomes in liver resection for patients with comorbid cirrhosis. However, this is not an affirmation of LH for all cases. In LH, there is restricted operation with forceps, and sufficient training is required to deal with the difficulty of setting detailed liver resection lines and expanding the field of view. Procedures associated with vascular reconstruction can be performed only in a limited number of facilities. There are still many issues associated with this procedure, including the need for caution in patients with comorbidities, such as heart disease, and it will take some time for these issues to be resolved effectively.
Conclusions
LH was not different compared to OH in terms of the operative time and incidence of postoperative liver failure in patients comorbid with cirrhosis, who have insufficient hepatic reserve. However, LH was associated with significantly lower perioperative blood loss, the incidence of postoperative complications (such as refractory ascites and pleural effusion), and operative mortality rate. Still, the number of cases in this study was small, and the study may have had several biases, including the location of the tumour, particularly with respect to the blood vessels; thus, it is difficult to claim that there is a high degree of evidence. Further randomised controlled trials and meta-analyses are needed.









