Although proton pump inhibitors (PPI) remain the mainstay of peptic ulcer disease (PUD) treatment, their role in severe reflux esophagitis is less significant. It is because of multifactorial causes of gastroesophageal reflux disease (GERD) with acid toxicity being only one of them. The healing process takes time which is important in severe cases when rapid healing is crucial in the prevention of complications.
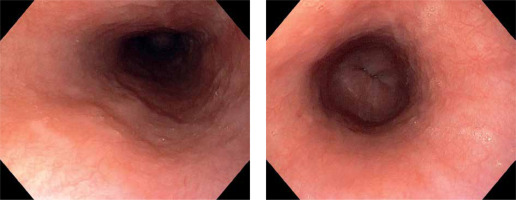
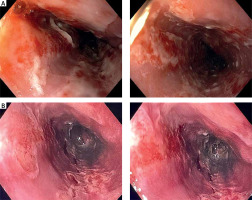
Thus we present a 62-year-old male, who presented to the emergency department with abdominal pain, heartburn, nausea and vomiting, and lastly with blood. Symptoms started after he had been treated with non-steroidal anti-inflammatory drugs for acute viral infection for three days. His past history was relevant for hypertension and type 2 diabetes. Laboratory tests were within normal ranges. Urgent gastroscopy revealed a large duodenal ulcer and severe esophagitis with a circumferential ulcer 15 cm in length (Figure 1 A). During the procedure further mucosal damage with bleeding caused by severe nausea and vomiting occurred. We hypothesized that the most probable cause of nausea and vomiting leading to esophagitis with impending perforation was functional sub-occlusion in the course of PUD. The intravenous proton pump inhibitor was implemented at a dose of 40 mg b.i.d. and also we added an Esoxx sachet three times daily. Prophylactically, the patient received antiemetic drugs. At first fluid diet was allowed, and in the following days, the diet was extended. After 4 days we decided to perform a second look gastroscopy with the intention of an intervention procedure in case of threatening duodenal ulcer bleeding. There has been significant improvement concerning ulcer disease. Surprisingly also esophageal ulcers were almost healed with much shallower erosions with filling epithelium. There were no signs of active or past bleeding (Figure 1 B). Endoscopy performed after 6 weeks confirmed sustained improvement (Figure 2).
Figure 1
A – Esophageal changes before treatment. B – Improvement of esophageal changes after 4 days of treatment
Such rapid mucosal healing was unexpected given the efficacy data of PPIs. The largest meta-analysis showed that the weekly percentage of mucosal healing is 11.5%, which means that almost 90% of patients after a week of PPI use still have active inflammatory changes in the esophagus [1]. In a prospective multicenter analysis, it was proved that in grades C and D, esophagitis (LA classification) heals after 8 weeks only in 67.5% of cases [2]. Therefore, the reason for such rapid improvement and healing was considered to be the effect of adding Esoxx to the treatment. It is consistent with results of a recent study assessing symptom relief and mucosal healing in patients with radiation esophagitis [3]. Authors found that more than 92% of patients experienced symptoms’ improvement which lad to completion of oncologic treatment without delay.
Esoxx is a topical agent acting protective and healing esophageal mucosa. It is a combination preparation containing chondroitin sulfate (SC) and hyaluronic acid (HA) suspended in a bioadhesive carrier adapted to adhere to the esophageal mucosa – poloxamer 407 [4]. Esoxx makes a complex protective layer covering the mucosa, constituting a mechanical protective barrier against irritants, which has been proved in experimental and clinical studies [5]. It has a liquid form, easy to apply, in specially prepared sachets with one dose of the drug. It is not absorbed from the gastrointestinal tract, and therefore is safe for the patient. It does not interact with drugs or food.
In conclusion, the example discussed above shows that the addition of Esoxx to the treatment of esophagitis improves the effectiveness of therapy and shortens the healing time of the mucosa.