Introduction
Endoscopist-directed nurse-administered sedation (EDNAS) using propofol and fentanyl in patients undergoing endoscopic retrograde cholangiopancreatography (ERCP) is being increasingly utilized worldwide. However, this method of sedation is not usually employed in India by endoscopists due to concerns surrounding its efficacy and safety. Propofol was introduced in the 1980s. It has become well-known for moderate sedation use for GI endoscopic procedures and its use has been increasingly adopted during the past decade [1]. It provides additional benefits including reduced time to induction of sedation, shorter duration of action, faster patient recovery time, higher post-procedure patient satisfaction, and greater patient willingness to repeat endoscopy in the future [2]. Propofol-based sedation has a similar rate of adverse events to other forms of sedation. It can be safely administered with fixed doses of benzodiazepines and/or opioids to enhance hypnotic and sedative effects [3]. The combination of propofol with other sedative agents leads to a reduction in the propofol dose, thereby improving its safety profile [4]. This moderate sedation regimen is known as “balanced propofol solution” (BPS). In BPS, a benzodiazepine, propofol, and an opioid are administered in a single dose. This is then complemented by small incremental doses of propofol (10–20 mg/push) to obtain a target level of moderate sedation [5]. However, higher doses of propofol can induce deep sedation leading to respiratory depression and apnoea [6]. Furthermore, there is currently no known propofol antagonist. Moreover, propofol product labelling states that “propofol should be administered only by persons trained in the administration of general anaesthesia” [7]. Unfortunately, there are few anaesthetists in developing countries, particularly in India.
No major study regarding EDNAS using propofol with midazolam/fentanyl for ERCP from India is available.
Aim
Hence, the aim of this study was to assess the efficacy, acceptability, and safety of this type of sedation in low to moderate risk patients undergoing ERCP in a developing nation like India where resources are limited for general anaesthesia.
Material and methods
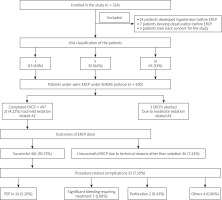
This study was conducted at a single tertiary hospital in Paras Hospital, Gurugram, India. It was a prospective study involving 500 patients with an ASA score of I–III, presenting with any indication for ERCP. Informed consent was taken from all patients. A total of 500 patients (348 male and 152 females) with a mean age of 57.3 years (range: 20–87) were recruited from August 2016 to July 2019. The following patients were excluded:
Those who did not consent for the study.
Those in ASA class IV and V.
Those with difficult airways, e.g. with high Malampati score, short neck, obesity, etc.
Those with a history of failed ERCP or non-cooperation or requiring double stenting.
Psychiatric or irritable patients.
Those below 18 or above 70 years of age (Figure 1).
The sedation was given by the trained nurses posted in the endoscopy OT. Intra-procedural and post-procedure (AEs) adverse events due to sedation were recorded by the doctors. The endoscopist was also responsible for directing the provision and dosing of the BPS. Complete cardiopulmonary resuscitation equipment and medications were available within the endoscopy unit. Endoscopy doctors and nurses were necessarily basic and advanced cardiac life-support certified.
In this study, we evaluated patient-level demographic variables including age, gender, ASA classification, and indication for ERCP. We also evaluated clinically relevant patient outcome variables including the following: BPS drug dosages, pre-procedure and post-procedure oxygen saturation levels, pulse, systolic/diastolic blood pressures, and the need for mask bag ventilation or endotracheal intubation at any time during or immediately following ERCP. Aborted ERCP procedures due to a sedation-related adverse event, any ICU admission, and mortality were also recorded. Institutional Ethics Committee approval was obtained for this study.
Statistical analysis
Descriptive statistics for all parameters were calculated: For continuous variables this included mean, standard deviation (SD), median, minimum, maximum, and range values. For non-continuous variables, counts and percentages were reported. A previously determined value of p < 0.05 was considered statistically significant.
Results
From 1 August 2016 to 30 October 2018, 500 patients undergoing ERCP received endoscopist-directed sedation.
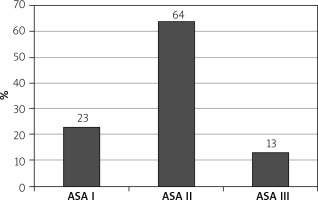
Among them 150 (30%) were alcoholics, 225 (45%) were smokers, 348 (69.6%) were males, 152 (30.4%) were females (Table I), 115 (23%) were American Society of Anaesthesiologists (ASA) I, 320 (64%) were ASA II and 65 (13%) were ASA III with PS 1–2. The mean age was 57.3 years (Figure 2).
Table I
Demographics
| Parameter | N | % |
|---|---|---|
| ASA I | 115 | 23 |
| ASA II | 320 | 64 |
| ASA III | 65 | 13 |
| Males | 348 | 69.6 |
| Females | 152 | 30.4 |
| Alcoholics | 150 | 30 |
| Smokers | 225 | 45 |
| Choledocholithiasis | 415 | 83 |
| Benign CBD stricture | 45 | 9 |
| Malignant OJ | 30 | 6 |
| Bile duct injury | 10 | 2 |
Indications for ERCP by the endoscopist-directed, nurse-administered (EDNA) sedation included 415 (83%) choledocholithiasis, 45 (9%) benign CBD strictures, 30 (6%) malignant obstructive jaundice (OJ), and 10 (2%) bile duct injuries (Table II).
Table II
Results of endoscopist direct nurse administered sedation with balanced propofol solution in ERCP
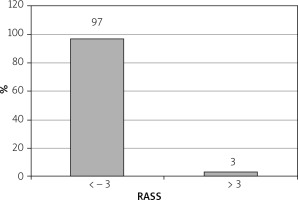
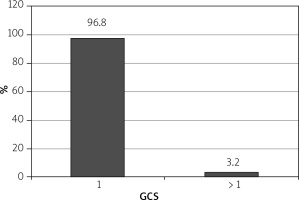
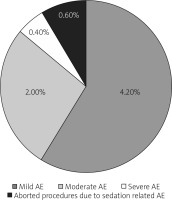
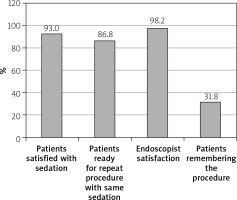
Sedative dosages (mean ± standard deviation) per patient were propofol = 90 ±20 mg, fentanyl 0.75 ±0.25 mg (range: 0.25–1.00 mg), and midazolam 2 ±0.5 mg (range: 1–3 mg). Mean injection to sedation time was 2 min (range: 1–4 min). 97% of patients achieved a Richmond agitation sedation score (RASS) of ≥ –3 (Figure 3) and 96.8% achieved a Gloucester comfort score (GCS) of ≤ 2 (Figure 4) during the procedure. 4.22% of the patients had mild adverse events and 2.11% had moderate AE in the form of nausea, hypotension, breathing difficulty, or hypoxemia. Two (0.4%) patients required intubation and ICU admission. The remaining patients resolved all after conservative management in the recovery room itself. There were 3 (0.6%) aborted ERCPS due to moderate sedation-related AE. There was no mortality secondary to sedation-related AE (Figure 5). The mean recovery time was 15.3 min. 98.3% of the endoscopists were satisfied with the sedation achieved during procedure. 31.2% of the patients remembered the procedure. 93% of the patients were satisfied with the type of sedation, and 86.8% were ready for a repeat procedure, if indicated, under the same sedation (Figure 6).
The outcomes of ERCPs: Among 497 ERCPs done in patients under EDNAS, 461 (92.75%) were successful and 36 (7.24%) were unsuccessful due to technical reasons other than sedation related. 35 (7.59%) had procedure-related complications: in the form of post-ERCP pancreatitis (PEP) in 24 (5.290%); significant bleeding requiring treatment in 5 (1.08%); perforation in 2 (0.43%); and others like ascending cholangitis in 4 (0.86%) patients. However, none had procedure-related mortality.
Discussion
There is ongoing controversy and lack of consensus regarding the safety and efficacy of endoscopist-directed BPS in the absence of monitored anaesthesia care during GI endoscopy procedures because it has a narrow therapeutic index and can cause irreversible respiratory depression.
No major study regarding EDNAS using propofol with midazolam/fentanyl for ERCP from India is available. Our study is one of the first such studies. We selected about 500 patients with ASA score of I to III. The majority of our patients had moderate risk (ASA II) due to comorbidities/jaundice.
The mean dosage of propofol was 90 ±20 mg, fentanyl 0.75 ±0.25 mg, and midazolam 2 ±0.5 mg, which is less than used by Nathan et al. [8]. However, Clarke et al. evaluated 15,268 colonoscopies in which the mean propofol dose used was 60 mg, administered in combination with midazolam and fentanyl [9].
Our sedation protocol showed high efficacy; 97% of our patients achieved Richmond agitation sedation score (RASS) of ≥ –3.
Mean recovery time was 15.3 min. This is lower than achieved by Sathananthan et al. in their study (23 min) [10]. Rapid recovery and return to baseline behaviour allowed us to quickly discharge patients or move them to the ward from the endoscopy unit after the procedures. This saved a lot of time and increased the number of procedures done per day.
4.22% and 2.11% of the patients had mild and moderate AE, respectively, in the form of hypotension, breathing difficulty, bradycardia, or hypoxaemia. 0.4% had severe AE and required intubation and ICU admission. 0.6% had aborted ERCPS due to moderate sedation-related AE. These percentages of AE are much lower than in previous studies. Quaid et al. reported 2–16% of sedation related AE [11]. Rex et al. reported in their study that 0.1% of their patients needed bag and mask ventilation, 11 patients required intubation, and 4 patients died [12].
98.3% of the endoscopists were satisfied with the sedation achieved. This is the same as seen in other studies. Physician satisfaction with propofol sedation was about 95% in 2 colonoscopy studies as reported by Ulmer et al. and Hansen et al. [13, 14].
The mean recovery time was 15.3 min. This is lower than that achieved by Sathananthan et al. in their study (23 min) [10].
31.2% of the patients had some memory of the procedures done to them, which is less than with midazolam or midazolam/fentanyl combination. Two colonoscopy trials found that there was 13% more memory of the procedures sedated with midazolam/narcotics than with propofol and 10% fewer were satisfied with their sedation [11].
93% of the patients were satisfied with the type of sedation, and 86.8% were ready for a repeat procedure, if indicated, under the same the sedation. The same results were shown by other studies.
Endoscopist-directed BPS may have substantial economic advantages; the financial burden of anaesthesiologist services for GI endoscopic procedures is estimated to be $5 billion annually in the USA [15]. Furthermore, a lot of time is saved using EDNAS. This will increase the number of endoscopic procedures that can be done in the hospital. This is very important in a country like ours where the demand always exceeds the availability.
The outcomes of ERCP under EDNAS are similar to those done under anaesthetist-directed sedation with lower or similar complication rates [16]. Hence, our study proves that endoscopist-directed sedation does not affect the efficiency of the doctor.