Segmental resection of the distal duodenum is an attractive consideration in selected cases with isolated duodenal pathology. This can be either a local wedge duodenal excision or segmental resection, especially when there is no local infiltration and the ampulla of Vater can be preserved. Such an approach has the potential to avoid the morbidity and mortality risk of pancreatoduodenectomy [1–4]. However, it carries a 3.6% mortality rate and a high morbidity risk that must be considered [5]. Anastomotic leakage is one of most serious complications, the management of which remains of a challenge. The choice of treatment depends on the extent of breakdown, viability of duodenal margins at the defect and the degree of peritoneal contamination, which is largely dependent on the timing of the diagnosis of a leak [6, 7]. Pyloric exclusion with biliary diversion is a well-established rescue treatment for high-risk duodenal perforations [6, 8]. Proper surgical technique including precise suture placement, no tension at the anastomosis and well-vascularized anastomotic edges are three well-known prerequisites for optimal healing. Any compromise in these factors increases the risk of anastomotic failure.
Herein we report on a case of an extremely complicated postoperative course with dehiscence of duodenojejunostomy following a limited resection within the fourth portion of the duodenum (D4) together with the first part of the jejunum for a duodenal neuroendocrine tumour (NET). We also try to ascertain the cause of the dehiscence of anastomosis in our patient and our management strategy in this case and propose an alternative approach to prevent repetition.
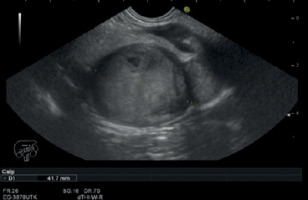
A 45-year-old female patient presented with epigastric pain, weight loss and symptoms of partial gastric outlet obstruction. An abdominal computed tomography (CT) scan revealed a solid tumour consisting of two components, measuring 35 × 36 mm and 20 × 24 mm, located between the distal duodenum, pancreas and aorta, enhancing in the arterial phase (Figures 1 A, B). An endoscopic ultrasound scan confirmed a well-demarcated and hypoechoic subepithelial duodenal lesion of homogeneous pattern with regular margins and a focus of cystic necrotic degeneration (Figure 2). Given the significant bowel obstruction, surgery was indicated without prior biopsy.
Figure 1
A – Coronal computed tomography (CT) arterial phase image demonstrating two adhering components of duodenal NET. Pronounced homogeneous enhancement of the upper component (long arrow) and moderate enhancement of the lower component (short arrow). B – Axial CT arterial phase image showing non-homogeneous and only moderate enhancement of the lower component of duodenal NET (arrow)
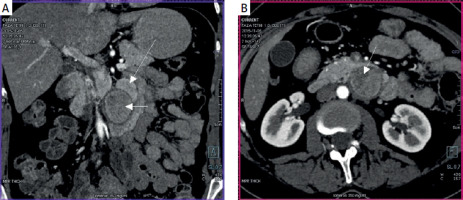
Figure 2
Endoscopic ultrasound (EUS) image of subepithelial hypoechoic duodenal tumour showing regular, well-demarcated margins and focus of cystic necrotic degeneration (arrow)
The radiological features indicated a NET of the duodenum as the most likely diagnosis. Laboratory tests showed no significant abnormalities, with normal values of tumour markers and normal chromogranin A level. The patient consented to distal duodenal resection or pancreatoduodenectomy, depending on the intraoperative findings.
At surgery, following a Kocher and Cattel-Braasch manoeuvre, and division of the ligament of Treitz, a tumour originating from the fourth portion of the duodenum (D4) was exposed and successfully dissected off the pancreas (Figure 3 A). As the superior mesenteric vessels were not involved, a limited resection of most of D4 and the first jejunal loop was performed. A tension-free duodenojejunal anastomosis was performed using long-lasting absorbable monofilament sutures (Figure 3 B). Histopathological examination confirmed the diagnosis of a low-grade, well-differentiated NET (Ki67 < 5%).
Figure 3
A – Duodenal intramural NET of the fourth portion of the duodenum (D4). The duodenum is divided into four parts: the duodenal bulb (first portion or D1), descending part with genu inferius (D2), the horizontal segment located to the right of the superior mesenteric vessels (D3) and the fourth portion (D4), which is on the left of the superior mesenteric axis. B – Duodenojejunal anastomosis between the proximal D4 (close to the mesenteric root) and the “new first jejunal loop” following a limited resection of D4 with a part of the jejunum. C – Distal duodenal exclusion with cholecystojejunostomy, gastrojejunostomy and Brown’s side-to-side jejunojejunostomy. Duodenal closure with mechanical stapler was applied at the proximal part of D3 and just below the genu inferius (arrow)
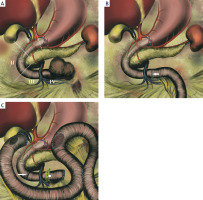
Eight days postoperatively, the patient developed diffuse peritonitis requiring emergent relaparotomy. Major anastomotic dehiscence (around 50% circumference) with intra-abdominal contamination was found. Due to suspected ischemia, we opted for distal duodenal exclusion to divert bile and gastric secretions away from the leakage site. The original plan for pyloric exclusion with Roux-en-Y hepaticojejunostomy was abandoned due to limited mesenteric mobility. Instead, we performed cholecystojejunostomy, distal duodenal exclusion, and gastrojejunostomy with Brown’s side-to-side jejunostomy (Figure 3 C).
Postoperatively, the patient developed massive intra-abdominal bleeding (day 13), requiring multiple relaparotomies with retroperitoneal gauze packing. Bleeding recurred after pack removal (days 20, 21), necessitating an “atraumatic” gauze technique using antibacterial foil wraps, which successfully prevented further haemorrhage.
A pancreatic fistula developed postoperatively, draining 200–300 ml/day, but resolved over 10 weeks with conservative management, including enteral feeding and somatostatin analogues. The patient was discharged home after 107 days, having undergone six relaparotomies, and remains well at 9 years follow-up with no tumour recurrence.
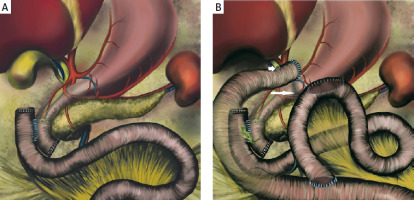
The duodenum is a C-shaped retroperitoneal organ (except for the duodenal bulb) with no mesentery, that extends from the pyloric sphincter to the ligament of Treitz. Anatomically it is divided into four sections including the bulb (first portion or D1), the descending part with the inferior flexure (D2), the horizontal part (third portion or D3) and the ascending duodenum (D4) [9]. D2 is the part where the main bile duct and the main pancreatic duct typically open into the duodenal lumen through the ampulla of Vater. The horizontal segment of D3 is located to the right of the superior mesenteric vessels, while D4 is the last portion, on the left of the superior mesenteric axis (Figure 3 A). The vascularization of D2 and the proximal part of D3 is intimately linked to the vascular supply of the pancreas from the superior and inferior pancreaticoduodenal arteries arising from the gastroduodenal and superior mesenteric arteries, respectively. This is believed to be the best site for the anastomosis, while blood supply to the distal portion of D3 and D4 is tenuous and assured only by the collateral branches of the pancreaticoduodenal arcades and the initial left branches of the superior mesenteric artery [10, 11]. In 25% of cases, D4 lacks a dedicated vascular supply (“watershed area”), increasing the risk of anastomotic failure [12]. This may create a significant risk for poor anastomotic healing, which was probably the main cause of anastomotic dehiscence in our patient. Despite a tension-free anastomosis performed by an experienced hepatobiliary surgeon, the patient developed anastomotic breakdown with a cascade of complications later on, confirming the high failure rate of D4 anastomoses [10, 12, 13]. To ensure adequate perfusion, resection should always include D3, D4 and the proximal jejunum, avoiding direct D4 anastomoses [14, 15]. However, this may be a difficult procedure mainly due to the anatomic location of the superior mesenteric axis. Following Kocherization along with the Cattell-Braasch manoeuvre and ligament of Treitz division, the D3, D4 and the first limb of the jejunum need to be carefully resected, paying special attention not to cause any damage or injuries to the superior mesenteric vessels. When the resection is completed, the remaining jejunum is passed in front of the mesenteric root towards the right side. Performance of the anastomosis with the jejunum as high as possible – that is, at the lower part of the D2 infra-ampullary portion – may further reduce the risk of compromised regional blood supply. However, the ampulla of Vater needs to be localized first, so as not to be jeopardized or injured during surgery. Digestive continuity may be restored by end-to-end or side-to-side duodenojejunostomy (Figure 4 A) [16]. Should this end up with anastomotic leakage, pyloric exclusion with biliary diversion achieved either via T-tube external biliary drainage or Roux-en-Y hepaticojejunostomy (Figure 4 B) may be the best rescue strategy in difficult cases [6–8, 17].
Figure 4
A –Side-to-side duodenojejunostomy following pancreas-sparing resection of D3 and D4 with proximal jejunum. B – Pyloric exclusion with Roux-en-Y hepaticojejunostomy (short arrow) and gastrojejunostomy performed for a leakage at the side-to-side duodenojejunal anastomosis. The pyloric ring may be easily closed from inside the stomach with a running suture (long arrow) following gastrotomy (close to the pylorus) and prior to creating gastrojejunostomy
The surgical technique we have chosen to treat dehiscence of duodenojejunostomy in our patient was based on closure of the proximal part of D3 with a mechanical stapler (Figure 3 C – long arrow) followed by the formation of cholecystojejunostomy and gastrojejunostomy to direct all gastric and biliary secretions away from the distal duodenum (Figure 3 C). As an alternative, we also considered performing total pancreatoduodenectomy with splenectomy. However, such an extensive surgery in a patient with diffuse peritonitis was considered too risky. Despite a very complicated postoperative course, duodenal exclusion with biliary diversion resulted in complete recovery of our patient and good long-term outcome.
In conclusion, this case highlights the high failure risk of D4 anastomoses due to tenuous blood supply. For tumours at D3/D4, complete resection with proximal jejunum removal is recommended. If leakage occurs, duodenal exclusion with biliary diversion is an effective salvage approach.









