Ampullary adenocarcinoma (AC, cancer of the papilla of Vater) comprises 0.3% of gastrointestinal tumors. Up to 50% of patients with AC are diagnosed at the stage of unresectable or metastatic disease [1]. Patients with disseminated disease are referred for palliative chemotherapy [2]. There are no guidelines on the management of oligometastatic disease, widely understood as a process limited to 5 metastatic lesions [3]. Liver metastasectomy may be performed in selected patients with colon and neuroendocrine cancers. Data on long-term survival in patients undergoing metastasectomy in pancreato-biliary cancers are contradictory, and there is not sufficient evidence to introduce that procedure in periampullary tumors [3].
Herein, we present a case of liver metastasectomy, performed in a patient with ampullary cancer and metachronous oligometastatic liver disease.
The 70-year-old male patient self-referred to the emergency department with jaundice, persistent pain in the epigastric region, and weight loss of 10 kg over 3 months. Gastroscopy revealed a 35-mm ulcerated ampullary tumor. Histopathological examination of the tumor biopsy revealed a high-grade ampullary adenoma with limited AC foci. After abdominal computed tomography (CT), which did not visualize metastases, the patient was referred by a multidisciplinary team for surgical treatment. The preoperative CA 19-9 serum concentration was 443 U/ml. The patient underwent pancreaticoduodenectomy followed by digestive tract reconstruction using the Traverso technique (Figures 1 A–D). In the perioperative period, no complications were reported. Histopathological examination of the surgical specimen revealed a 35-mm poorly differentiated AC. The histological type of cancer was mixed, with a predominance of pancreato-biliary type over intestinal type (Figures 2 A, B). Desmoplastic tumor stroma was heavily infiltrated by inflammatory cells, mainly neutrophils. Cancer cells invaded the ampulla, Oddi sphincter, duodenal wall, pancreatic head, as well as adjacent fat tissue. Perineural invasion (Figure 2 B) and lymph vascular invasion were present. Tumor cells diffusely expressed cytokeratin 7 (Figure 2 C). Less than 20% of tumor cells expressed cytokeratin 20 (Figure 2 D). Expression of the intestinal marker CDX2 was focal (not shown). Negative surgical margins (R0) were achieved, but tumor cells were found at a close distance (less than 1 mm) from the posterior circumferential (radial) margin. Six metastatic lymph nodes were found among a total of 29 examined lymph nodes. The stage of the disease was assessed as pT3bN2cM0, according to the 8th edition of the American Joint Committee on Cancer criteria for AC. Contrast-enhanced CT performed 1 month after the surgery revealed a solitary, hypovascular metastatic lesion, measuring 34 × 32 mm, located between the 6th and 7th liver segments (Figure 3 A). 18F-fluorodeoxyglucose positron emission tomography-CT (18F-FDG PET-CT) imaging confirmed a single tumor with increased glucose uptake, consistent with a liver metastatic lesion, without any other metastatic foci (Figures 3 C, D). Considering the surgical resectability of the metastatic lesion, prior R0 pancreaticoduodenectomy, and good performance status – Eastern Cooperative Oncology Group (ECOG) 1 – neoadjuvant chemotherapy and subsequent metastasectomy were advised by a multidisciplinary team. Neoadjuvant chemotherapy with five courses of cisplatin 25 mg/m2 and gemcitabine 1000 mg/m2 was administered on the 1st and the 8th day of every course. After four courses of chemotherapy, a significant reduction of metastatic lesion to a size of 12 × 15 mm was observed (Figure 3 B). After the 5th course of chemotherapy, a non-anatomical resection of the liver lesion was performed. Histopathological examination revealed an 8-mm adenocarcinoma, consistent with metastasis of the AC (Figure 3 E). Expression of cytokeratin 7 in tumor cells was diffuse (Figure 3 F), but cytokeratin 20 was not expressed. Adjuvant chemotherapy with 5-fluorouracil and cisplatin was proposed, but the patient refused any further treatment. Follow-up CT, performed 3 months after surgery, revealed no recurrence of the disease. At the time of the follow-up, the disease-free survival (DFS) was 55 months.
Figure 1
Pancreaticoduodenectomy with digestive tract reconstruction using Traverso technique. A, B – after removal of the specimen – superior mesenteric artery (SMA), common hepatic artery (CHA), abdominal aorta (Ao), portal vein (PV), superior mesenteric vein (SMV), splenic vein (SV), left renal vein (LRV), inferior vena cava (IVC). C – reconstruction – duct-to-mucosa pancreatico-jejunostomy – main pancreatic duct (MPD). D – reconstruction – duodenojejunostomy
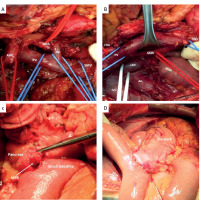
Figure 2
Histopathological examination. A – Ampullary adenocarcinoma – primary tumor. Desmoplastic tumor stroma heavily infiltrated by inflammatory cells, mainly neutrophils. Original magnification: 10×. B – Ampullary adenocarcinoma – primary tumor. Extensive perineural invasion. Original magnification: 10×. C – Ampullary adenocarcinoma – primary tumor. Cytokeratin 7 expression. Original magnification: 10×. D – Ampullary adenocarcinoma – primary tumor. Cytokeratin 20 expression. Original magnification: 10×
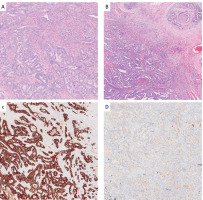
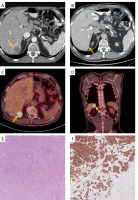
Figure 3
Imaging of the metastatic lesion and histopathological examination. A, B – Contrast-enhanced CT. Solitary hypovascular metastatic lesion between the 6th and 7th liver segments. A – before neoadjuvant treatment – lesion measuring 32 × 35 mm (yellow arrowhead). B – significant reduction of metastatic lesion to a size of 12 × 15 mm after neoadjuvant treatment (yellow arrowhead). C, D – 18F-FDG PET-CT. Solitary lesion between the 6th and 7th liver segments, with increased FDG uptake, consistent with a liver metastatic tumor (yellow arrowheads). E – Ampullary adenocarcinoma – liver metastasis. Liver parenchyma is visible in the lower right of the image. Original magnification: 10×. F – Ampullary adenocarcinoma – liver metastasis. Cytokeratin 7 expression. Original magnification: 10×
The case described above introduces an unconventional approach to the treatment of oligometastatic, pancreaticobiliary type AC, in which resection of metachronous liver metastasis resulted in a favorable outcome with long disease-free survival. Histologically, AC represents a heterogenous group of gastrointestinal tumors, classified as pancreaticobiliary (Pb-AC), intestinal (Int-AC) and mixed (Mixed-AC) types. Pb-AC and Mixed-AC histotypes are independently associated with an advanced tumor stage, an increased rate of lymph node involvement, as well as perineural and lymphovascular invasion [4]. Patients who have undergone pancreaticoduodenectomy with Pb-AC or Mixed-AC in stages IIB-II exhibit notably reduced median overall survival (mOS) and disease-free survival (DFS), along with an elevated recurrence rate when compared to those with Int-AC [4]. The efficacy of liver metastasectomy in the OS improvement in patients with oligometastatic periampullary tumors is based on limited studies and remains controversial, both in terms of eligibility criteria and the use of chemotherapy before and after surgical treatment [5]. Considering literature data on liver metastasectomy in pancreatic ductal adenocarcinoma (PDAC), median overall survival ranges from 5.9 to 54.6 months following resection of metastasis [6]. It is worth pointing out that in most of the analyzed cases, the mOS does not exceed 15 months. According to some studies, liver metastasectomy in PDAC may be comparable to palliative chemotherapy in terms of overall survival [7]. Takeda et al. reported 10 cases of highly selected patients with PDAC treated by liver metastasectomy, who achieved a long, median OS of 54.6 months. It is worth noting that patients were strictly selected for the study and had to meet at least 3 of 4 criteria including age < 70, good performance status of ECOG 0, a modified Glasgow score of 0, and Ca19-9 values below < 1000 U/ml. It should be stressed that the individuals as mentioned above represent a significant minority of PDAC patients, making it difficult to relate mOS values to the general population of patients undergoing liver metastasectomy for PDAC [8]. De Jong et al. conducted a study that involved the comparison of liver metastasectomy in 40 patients with histologically confirmed periampullary cancers, including 30 Pb-ACs and 10 Int-ACs. The percentage of 3-year survival as well as mOS in patients with Int-ACs were significantly higher compared to the cohort with Pb-ACs (33% vs. 8%, p = 0.05, and 23 months vs. 13 months, p = 0.05), respectively. The authors observed significantly longer survival in patients undergoing metastasectomy for intestinal-type tumors, compared to pancreaticobiliary type (13 months vs. 18 months, p = 0.05) [7]. Resectability criteria for liver metastatic tumors remain vague; nevertheless, several authors tried to define resectability criteria for liver metastasectomy in periampullary tumors. The decision on eligibility for metastasectomy was based on the clinical stage of the disease, the patient’s performance status, prior R0 pancreaticoduodenectomy, acceptable liver function and the potential resectability of all metastatic lesions [7, 9]. The effect of neoadjuvant chemotherapy on improving overall survival in patients before radical treatment of the primary lesion is unclear; however, some recent studies have shown improved overall survival in patients undergoing PD for AC after neoadjuvant chemotherapy [2, 10]. Currently, no studies are treating the validity of neoadjuvant chemotherapy in patients with AC and oligometastatic liver disease. In our opinion, such treatment may be beneficial and result in a reduction of micrometastasis, allowing R0 resection to be achieved.
In conclusion, resection of liver metastasis may be beneficial in selected patients with ampullary adenocarcinoma AC and oligometastatic disease. Long-term survival is possible, especially in patients with a good performance status and liver metastasis responsive to neoadjuvant chemotherapy.










