Introduction
Acute liver failure (ALF), also known as ‘fulminant hepatic failure,’ is a rare and life-threatening condition [1, 2]. Acute liver failure accounted for 3.3% of liver transplants in adults in 2017 and 10.3% in the pediatric population in 2015–2017 [3]. The American Association for the Study of Liver Disease (AASLD) defines acute liver failure as the development of coagulopathy (INR > 1.5) and encephalopathy within 8 weeks of the first symptom in the absence of underlying cirrhosis. ALF patients can have an underlying condition for less than 26 weeks. Acute liver failure is further characterized into hyperacute (< 7 days), acute (7–21 days) and subacute (> 21 days) liver failure [4, 5]. The encephalopathy is differentiated between minimal and overt disease using West Haven criteria; grade III and IV encephalopathy are considered advanced HE [6, 7]. Drug-induced liver injury (DILI) accounts for 50% of ALF cases in the United States. Viral hepatitis remains the leading cause of ALF in developing countries. Other causes include ischemic hepatitis, Budd-Chiari syndrome, heat stroke, mushroom ingestion, and Wilson’s disease. In many cases, the cause of liver failure is unknown. These cases have a poor outcome without liver transplantation [8].
The survival rates in those who develop ALF have improved due to liver transplantation and improvement in intensive care management. There are no proven treatments for acute liver failure. Studies have shown that N-acetylcysteine (NAC) lowers the mortality, progression to stage III–IV hepatic encephalopathy, and end organ damage, and improves oxygen delivery and hemostasis in the acetaminophen overdose cohort [9, 10]. Although this benefit is not demonstrated in all of the studies [11], NAC is commonly used for the management of acute liver failure due to acetaminophen. The transplant-free survival of non-acetaminophen liver failure (NAI-ALF) is worse than acetaminophen-induced ALF [12]. The AASLD recommends the use of NAC in acute liver failure due to DILI and acetaminophen [4]. The European Association for Study of the Liver recommends use of NAC for both acetaminophen- and non-acetaminophen-induced acute liver failure [13]. However, studies published to date have not shown convincing benefits with NAC in NAI-ALF.
Aim
In the present systematic review and meta-analysis, the aim is to pool the available published data to further determine the safety and efficacy of NAC in non-acetaminophen-induced acute liver failure.
Material and methods
The study complies with Preferred Reporting Items for Systematic review and Meta-Analysis guidelines [14]. The study was considered exempt by our institutional review board since de-identified data were used.
Database search
The PubMed, Medline, Google Scholar, and Cochrane databases and clinicaltrial.gov were searched. A combination of the following keywords was searched in English: acetylcysteine and non-acetaminophen/non-paracetamol, drug-induced liver failure, viral hepatitis, or autoimmune hepatitis-induced ALF. In addition, we searched the references of the selected articles to find related articles that were not identified by the electronic searches. Pertinent studies were initially searched based on the title and the abstract, then the full text was read to verify the relevance. Two investigators did the search blindly (WA and FA) and the third investigator (WQ) resolved differences after the discussion.
Inclusion and exclusion criteria
We used the following criteria: prospective studies with a control group which looked at outcomes of non-acetaminophen liver failure in adults who received NAC. Length of follow-up was from 3 weeks to 6 months. Studies on liver failure due to alcohol use were excluded because the NAC treatment was compared with prednisone. Also, most cases of alcohol-related liver failure present as acute-on-chronic liver failure (ACLF), as shown in a large, single-center study [15]. We included only acute liver failure patients without underlying chronic disease. Studies in the pediatric population were excluded.
Primary and secondary outcomes
The primary outcome was overall mortality and secondary outcomes were transplant-free survival, safety of the NAC and length of hospital stay.
Data collection
The authors collected the following data: name of the author, year of publication, region, journal, type of study, number of subjects, mean age, sex, dosages of NAC, model for end-stage liver disease (MELD) score, encephalopathy grades, outcomes (survival, length of hospital stay, liver transplant, adverse effects), etiologies of liver disease. The authors of the published studies were contacted if data were missing.
Study selection
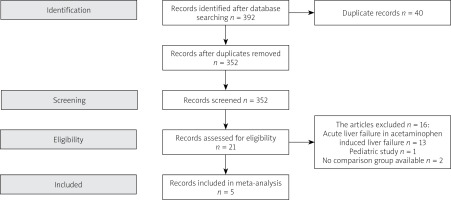
The studies were selected following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, which include screening, eligibility, included in systemic review or meta-analysis if applicable [14]. Duplicate studies and those which did not meet the inclusion criteria were excluded (Figure 1).
Statistical analysis
The results of all selected prospective studies were combined. The outcomes were presented as risk ratio with 95% confidence interval (CI). A p-value < 0.05 was considered statistically significant. To assess the quality of these studies, the guidelines presented by the Cochrane Handbook for Systematic Reviews of Interventions were followed. The funnel plot for asymmetry was used to assess the publication bias. The study demographics, clinical characteristics, event rates, and 95% CIs for the outcomes were extracted. We extracted risk ratios (RR) for NAC in non-acetaminophen ALF from published studies. The effect sizes were obtained from intention-to-treat analyses and fully adjusted models in the cohort studies. The primary analysis measured the pooled estimate of overall survival associated with NAC therapy.
To study heterogeneity, we hypothesized that the effect sizes might differ because of methodologic quality of the studies. Thus, we utilized a random effects model as described by DerSimonian-Laird [16] which assumes that the studies included in the meta-analysis are a random sample of hypothetical study populations. The random effects model estimates combined data with a wider CI, and the summary statistic is less likely to be significant. Heterogeneity was assessed using the Cochrane Q statistic, and the percentage of total variability due to true between-study heterogeneity was evaluated using the I2 measure. A p-value < 0.10 was considered significant for the I2 measure and interaction tests [17]. We assessed publication bias subjectively by visual inspection of Begg’s funnel plot [18] and objectively by Egger’s regression asymmetry test because funnel plots may be inaccurate in the assessment of very large studies [19, 20]. If the meta-analysis has captured all relevant studies, then the funnel plot is expected to be symmetric. All analyses were performed using Comprehensive Meta-Analysis v. 3 (Englewood, NJ) and STATA (College Station, Texas).
Results
Literature search
The initial search yielded 392 studies; after removing 40 duplicate studies, the authors thoroughly reviewed 352 records. Twenty-one prospective studies were identified. After using the inclusion criteria, 8 studies were selected. Two studies had no control group; one study involved a pediatric population. As a result, five studies were analyzed (Figure 1).
Systematic review of five studies
The details of the demographic and clinical characteristics of 5 studies were included in the analysis. The inclusion and exclusion criteria were identified. Two studies were randomized double blinded trials [21, 22] and three studies were prospective with a historical control [23–25]. Three studies were conducted in the United States and two were conducted outside the United States (Tables I, II). All five studies were included in the meta-analysis.
Table I
Characteristics of included studies
| Studies | Type of study | Region | Inclusion criteria | Exclusion criteria | Dose | Outcome |
|---|---|---|---|---|---|---|
| Nabi 2017 et al. [23] | Observational | South Asia | NAI-ALF ≥ 18 years old | Acetaminophen-induced ALF, acute on chronic liver failure, hepatic ischemia, ALF with pregnancy, previous exposure to NAC | IV: 150 mg/kg × 1 h, 12.5 mg/kg/h × 4 h, 6.25 mg/kg/h × 67 h | Survival, hospital length of stay |
| Darweesh 2017 et al. [24] | Observational | Middle East | NAI-ALF ≥ 18 years | Age < 18 years and > 70 years, liver failure due to cancer, previous exposure to NAC, recent use of acetaminophen, ischemic hepatitis | IV: 150 mg/kg in 0.5 h followed by 70 mg/kg in 4 h followed by 70 mg/kg in 16 h, then 150 mg/kg/day till two INR < 1.3 achieved. NAC was switched to PO after goal INR PO: 600 mg/day for 2–3 days after normalization of INR | Overall survival, transplant-free survival, length of stay |
| Singh et al. 2013 [21] | Double blinded | United States | NAI-ALF in individuals with ≥ 18 age | Previous NAC treatment, age > 70 years, undergoing immediate LT (< 6 h), refractory sepsis, cerebral edema, shock, ischemia, pregnancy, cancer | Intravenous NAC for 72 h (dose not specified) | 21-day overall survival, transplant-free survival |
| Lee et al. 2009 [22] | Randomized control trial, double blinded | Unites States | ALF in patients ≥ 18 year with underlying illness < 24 weeks | Age > 70 years, known or suspected acetaminophen use previously received NAC. Presence of pregnancy, cancer, liver ischemia, refractory hypotension, septic shock. Those who had undergone LT < 8 h | IV: 150 mg/kg × 1 h, 12.5 mg/kg/h × 4 h, 6.25 mg/kg/h × 67 h | Overall survival at 3 weeks, transplant-free survival and transplant rates |
| Mumtaz et al. 2009 [25] | Observational | South Asia | NAI-ALF | Use of acetaminophen, history of chronic liver disease | PO: 140 mg/kg × 1 h followed by 70 mg/kg, 17 doses 4 h apart | Overall survival, factors related to survival and safety of NAC in ALF patients and length of hospital stay |
Table II
Baseline characteristics of the studies included
| Study and year | Treatment groups (n) | Age (mean ± SD) or (median) | Sex (female %age) | Encephalopathy (III-IV) %age | MELD score median or mean | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| NAC N = 334 | Control N = 338 | NAC | Control | NAC | Control | NAC | Control | NAC | Control | |
| Nabi et al. 2017 [23] | 40 | 40 | 30.60 ±11.64 | 38.48 ±20.11 | 57.5 | 40 | 42.5 | 27.5 | 31.8 ±6.7 | 30.48 ±5.04 |
| Darweesh et al. 2017 [24] | 85 | 70 | 33.5 ±11 | 34.8 ±8.8 | 40 | 40 | 4.7 | 8.5 | NA | NA |
| Singh et al. 2013 [21] | 81 | 92 | 42 | 40.5 p = 0.68 | 68 | 47 | 73 | 62 | 32 | 33 |
| Lee et al. 2009 [22] | 81 | 92 | 42 | 40.5 p = 0.68 | 68 | 47 | 73 | 62 | 32 | 33 |
| Mumtaz et al. 2009 [25] | 47 | 44 | 27.7 ±11.8 | 37.52 ±18.82 | 44.7 | 45.5 | 68.1 | 45.5 | NA | NA |
Among these studies, 334 patients received NAC and 338 patients in the control group were analyzed. Viral hepatitis was the most common cause of the liver failure (45.8% vs. 32.8%) in the NAC and control group. The second most common cause in both groups was drug-induced liver injury followed by indeterminate cause and autoimmune hepatitis (Table III).
Table III
Etiologies of the acute liver failure in the included studies
| Studies | Indeterminate | DILI | Viral hepatitis | AIH | ||||
|---|---|---|---|---|---|---|---|---|
| NAC N = 44 | Control N = 73 | NAC N = 82 | Control N = 93 | NAC N = 153 | Control N = 111 | NAC N = 22 | Control N = 30 | |
| Nabi et al. 2017 [23] | 13 | 17 | 10 | 5 | 16 HEV = 7 | 14 HEV = 7 | ||
| Darweesh et al. 2017 [24] | 31 | 28 | 41 HAV = 12 HBV = 11 | 40 HAV = 10 HBV = 14 | ||||
| Singh et al. 2013 [21] | 15 | 26 | 19 | 26 | 25 HBV = 25 | 13 HBV = 13 | 11 | 15 |
| Lee et al. 2009 [22] | 15 | 26 | 19 | 26 | 25 HBV = 25 | 12 HBV = 12 | 11 | 15 |
| Mumtaz et al. 2009 [25] | 1 | 4 | 3 | 8 | 46 HEV = 24 HBV = 11 | 32 HEV = 16 HBV = 14 | ||
Overall and transplant-free survival
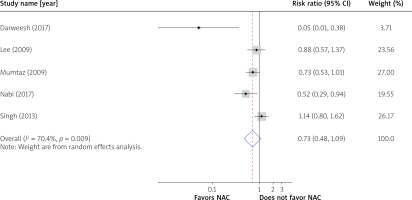
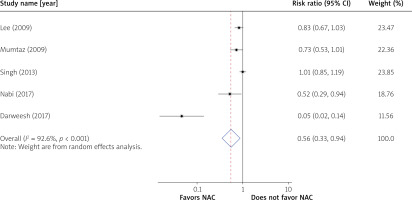
The overall survival was 70.1% (237/334) in the NAC group and 59.8% (202/338) in the control group (RR = 0.73; 95% CI: 0.48–1.09) (Figure 2). In the NAC group, the transplant-free survival was 55.1% (184/334) as compared to 28.1% (95/338) in the control group. The transplant-free survival was improved by 44% in the NAC group (RR = 0.56; 95% CI: 0.33–0.94) (Figure 3).
Bias assessment
There was heterogeneity in the assessment of the overall and transplant-free survival (I2 > 50%). Funnel plots were created for the bias assessment; asymmetry was noticed for the overall and transplant-free survival. A few studies on the right side of the plot were missed (Supplementary Figures S1 A, B). Statistically significant results are published more commonly and faster, which creates bias in the literature and can overestimate the effect of an intervention [26].
Duration of hospital stay
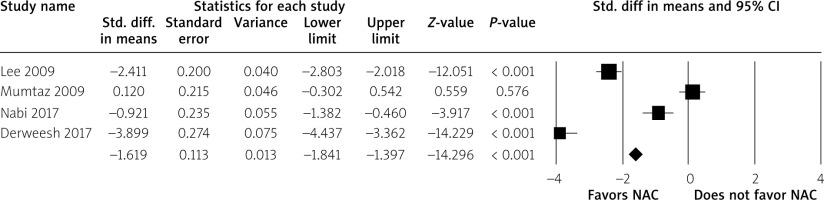
Out of five studies only four studies had data available for the duration of the hospital stay. The use of NAC significantly reduced the duration of hospital stay (SMD = –1.62; 95% CI: –1.84 to –1.40, p < 0.001) versus the control group (Figure 4).
Adverse events
The common adverse events in the NAC group were nausea, vomiting, dyspepsia, fever, rash, infections, arrhythmias and rarely bronchospasms. The adverse events were not statistically significant as compared to the control group in most studies (Supplementary Table SI). Prolonged cholestasis was seen in the NAC group in one of the studies, which was not observed in the control group [24].
Discussion
This systematic review of the existing published studies suggests that the transplant-free survival is improved with the use of NAC. The overall survival is not significantly improved after the treatment with NAC. The length of hospital stay among the survivors is decreased with the NAC treatment and the NAC treatment was relatively safe.
NAC was used as an IV infusion except in one study [25], where NAC was given orally. The treatment was given for at least 72 h in most of the studies. Except for increased incidence of nausea and vomiting in the NAC group in one study, the adverse events were not statistically significant [22].
The NAC thiol group can detoxify the free radicals and toxic metabolites of the acetaminophen and as a result limit the liver damage. It replenishes the glutathione stores in hepatocytes that combines with reactive agents and serves as the major antioxidant and protects hepatocytes from damage [27, 28]. NAC has also been shown to improve the tissue oxygenation and the hemodynamics in acetaminophen-induced liver injury [10]. The literature for support of NAC in non-acetaminophen-induced ALF is limited and heterogenous. One study suggests that NAC can improve the transplant-free survival by reducing the production of IL-17, which is a major proinflammatory cytokine [29]. The statistical significance was lost when overall mortality was considered. This might be because of confounding by the liver transplantation, which is the most effective management in these patients. Other possible causes are less advanced encephalopathy in the placebo group, and more viral hepatitis cases were found in the NAC group, which showed a relatively poor outcome [22, 30]. The better standard of critical care in both NAC and placebo groups can also improve the outcomes.
Only two of the analyzed studies did subgroup analysis on the DILI population. Nabi et al. [23] and Lee et al. [22] studies showed that use of NAC is associated with better overall and transplant-free survival in DILI. The Lee study also showed improved outcomes in patients with hepatitis B infection, whereas the former study did not show any survival benefit in ALF due to viral hepatitis. The NAC use in pediatric patients with acute liver failure did not show any benefit in one study. These results could be attributed to relatively different etiologies of liver failure [31].
The strength of our meta-analysis is the novelty of the study. There was one systematic review in the literature which reported similar results as ours [32]. The metanalysis is based on 3 prospective studies and one retrospective study. We did a comprehensive search; there is little likelihood that any eligible study was missed. The included studies are diverse, they were conducted in South Asia, the Middle East and the United States, and the results can be generalized.
Our study has a few limitations. First, the sample size of most of the included studies was small. Second, we did not have access to the patient data and used the pooled data from the prospective studies. Third, there were only 5 eligible adult studies and among adult studies the subjects were younger than 70 years old. The results cannot be generalized to all age groups. Fourth, only two of the studies included subgroup analysis for the etiologies of ALF; the exact outcomes of NAC in non-acetaminophen-induced liver failure based on etiologies is uncertain. Fifth, our study does not specify the benefit of NAC in early vs higher grade encephalopathy because of unavailability of data based on stage of coma. Two South Asian studies [23, 25] showed benefit of NAC in advanced hepatic encephalopathy, whereas Lee et al. [22] study did not show significant benefit in stage III–IV hepatic encephalopathy.
Conclusions
NAC demonstrated improved transplant-free survival and duration of the hospital stay in an adult population with non-acetaminophen-induced acute liver injury. The improvement in overall mortality is not statistically significant. More research is required to identify the role of NAC in both adults and the pediatric population with NAI-ALF.