Introduction
Type 1 diabetes (T1DM) is a chronic, uncurable, lifelong disease. It accounts for over 90% of all childhood and adolescent diabetes cases. In 2021, there were an estimated 108,300 children and adolescents aged less than 15 years newly diagnosed with T1DM, and 651,700 children and adolescents living with the condition worldwide [1].
Like many other chronic diseases, T1DM significantly affects the everyday functioning of the child and the whole family. Regardless of the treatment method, even including better and better use of modern technologies, from the day of the diagnosis, the life of the child and the family changes. As well as the need to perform additional, daily duties related to the treatment regime, children with diabetes and their parents bear a heavy burden of responsibility for therapeutic decisions and the risk associated with the constant risk of severe, acute complications of the disease. Hence, children and adults with T1DM are at significantly higher risk of psychological distress, anxiety, and depression than their healthy peers [2, 3]. This is confirmed by the results of research conducted in various parts of the world. Hood et al. in a self-report study revealed that nearly one in 7 adolescents with T1DM presented with signs of depression [4]. This level of depressive symptoms in children and adolescents with T1DM is nearly double that in youths in general [5]. According to the SEARCH study, approximately 9% of adolescents with diabetes suffer from moderate to severe depression and 14% present signs of its mild form [6]. Additionally, girls are more depressive than boys.
A recently published retrospective analysis of data from the Swedish nationwide registers [n = 20,005] showed elevated risk of depression, anxiety, and stress-related disorders in childhood-onset type 1 diabetes also after reaching adulthood. It was shown that among people who fell ill in childhood, the most common comorbidities were depression and anxiety. Moreover, relatives of individuals with T1DM were at elevated risk of developing these outcomes, with the highest risk seen in parents (aHRs 1.18–1.25), followed by siblings (aHRs 1.05–1.20) [2].
However, the results of research on the quality of life of adolescents with T1DM are somewhat surprising. In general, children with T1DM rate their quality of life as similar to that of their healthy peers [6], which is in contrast to the parents’ opinion, because they tend to rate their child’s quality of life lower [7, 8]. Some authors have also shown that girls with T1DM are more susceptible to the negative effects of the disease on the emotional and functional aspects of life [9]. Boys report better quality of life as well as youths with longer diabetes duration and those from a better socio-economic background [10, 11].
Some studies also analysed the impact of treatment outcomes and glycaemic control on the emotional state. Most of them show that poorer treatment outcomes are associated with a worsening emotional state [12, 13]. It was noticed that depression is more frequent in adolescents with T1DM with non-optimal glycaemic control, higher HbA1c values, and more frequent hospitalizations related to diabetes complications [14].
In recent years, tremendous advances have been made in the treatment of T1DM. The aim of modern methods of insulin therapy and glycaemic monitoring is not only better control of glycaemia, but also, most of all, to improve the quality of life of children with diabetes and their families by increasing safety and supporting therapeutic decisions in everyday life. However, attention should be paid to the possible negative aspects of striving for excellence. Perhaps a very strong focus on getting the best possible treatment results can also be a source of frustration and anxiety. Also, the use of modern devices itself may pose some limitations to the daily functioning of young people.
The aim of this study was to assess the prevalence of symptoms of depression and anxiety and estimate their potential association with various clinical parameters in adolescents aged 15–18 years with T1DM.
Material and methods
Participants
The study included 59 participants (age 15–18 years, mean 16.5; 59.3% male), and 59 of their parents. The coefficient of age variation was 6.2% (a variation coefficient less than 10% indicates insignificant variation in the value of the variable in the sample).
Research tools – psychological inventories
The study used validated questionnaires (Children’s Depression Inventory 2 [CDI2] and the State-Trait Anxiety Inventory [STAI], both in the Polish language version) [15, 16]. The set of CDI2 questionnaires include a set of diagnostic tools that provide a comprehensive assessment of depression symptoms in children and adolescents. The CDI2 allows the child to be desribed from the perspective of him/herself and the parent. It consists of a part filled in by the child and another separately by the parent [15]. The STAI is a tool for examining anxiety understood as a temporary and situationally conditioned state of an individual, and anxiety understood as a relatively permanent personality trait. It can be used for screening and individual diagnosis. It consists of 2 subscales, one of which (X1) measures state anxiety, and the other (X2) measures anxiety trait. The questions that make up both scales are placed on both sides of one test sheet. Each subscale consists of 20 items, to which the subject answers by choosing one of 4 categorized answers [16]. These questionnaires were completed separately by the parent and by the child. The method used to measure depression has a high reliability coefficient. Cronbach’s α-coefficient measuring the reliability of the children’s and parents’ results for the tested sample and subscales ranged between 0.75 and 0.87, which proves that the scale is sufficiently reliable for the tested sample [17]. A high level of anxiety X1, X2 defined has been defined for the value of the state scale: 8–10, and a high level of depressive symptoms was defined for the 10 scale values above 64. Additionally, the participants of the study were asked about subjective feelings about the influence of the disease on key aspects of their lives: relationship with peers (RELATIONS), learning results (RESULTS), way of spending free time (TIME), hobbies (HOBBY), and relations with siblings (SIBLINGS). The adolescents referred to each issue in the range from 1 to 5, where 1 and 2 meant that the disease did not affect the examined issues, and 4 and 5 – the disease had an impact on the examined factors. The middle answer was neutral, and it was difficult to express the sentence. Such an answer was classified more as a positive answer, which in the case of this study means that the disease did not influence the examined issues.
Medical data
The study also analysed the medical data regarding the course of the disease, such as the duration of T1DM, hospitalizations due to acute/chronic T1DM decompensation, and the presence of concomitant diseases. The current glycaemic control was determined by parameters obtained from at least 14 days of continuous glucose monitoring (SGM) or flash glucose monitoring (FGM) systems, and HbA1c. The ‘time in range’ (70–180 mg/dl) > 70% in adolescents using CGM and HbA1c ≤ 6.5% (48 mmol/mol) in the whole group were defined as good glycaemic control [18].
Statistics
All statistical calculations were performed using the statistical STATISTICA package from StatSoft Inc. (data analysis software system) version 13.3. The Shapiro-Wilk W test was used to check whether the quantitative variable was derived from a population with a normal distribution. The significance of differences between 2 groups in the model of unrelated variables was tested by Student’s t-test or the Mann-Whitney U-test (when the assumptions of the parametric test were not met). The significance of differences between 2 groups in the model of related variables was tested by Student’s t-test or Wilcoxon’s test (when the assumptions of the parametric test were not met). Correlation analysis was used to determine the relationship, strength, and direction between ordinal variables by calculating the Spearman correlation coefficient (r). Fisher’s exact test was used to examine associations for dichotomous characteristics and the Yulea coefficient (φ) as the measure of the strength of a relationship. Pearson’s chi-squared test for independence was used for categorical variables. The decision on the hypotheses to be verified was made by assuming a significance level of 0.05.
Results
Results of T1DM treatment
Approximately half of the participants were treated with a personal insulin pump (46%), and 70% constantly used CGM/FGM. The mean duration of T1DM was almost 6 years (range 1–15). Five (8.5%) of the participants in the previous year of the study required hospitalization due to diabetes: 2 due to severe hypoglycaemia and 3 due to insufficient glycaemic control. The mean value of TIR was 61.7% (range 15–100%). Thirty-four per cent of the study participants presented with TIR > 70%, and 39% with HbA1c ≤ 6.5%. Both HbA1c ≤ 6.5% and TIR > 70% were obtained by 24% (n = 14) of the participants. In the study group, differences in the mean HbA1c value were found between girls and boys (7.4 vs. 7.0%, respectively, p = 0.028). Girls using GCM/FGM also presented a lower mean TIR value (51.8 vs. 66.9%, respectively, p = 0.002). It was shown that optimal results of the T1DM treatment (defined as TIR > 70%, and HbA1c ≤ 6.5%) were not dependent on concomitant diseases (p = 0.447, φ = 0.06), participation in psychological therapy (p = 0.614, φ = 0.01), high anxiety level (X1) (p = 0.324, φ = 0.10), X2 (p = 0.503, φ = 0.04), high level of depression (p = 0.238, φ = 0.15), or emotional problems (p = 0.470, φ = 0.07). Interestingly, it was dependent on sex (p = 0.001, φ = 0.41) and on a high level of functional problems (p = 0.048, φ = 0.26).
Occurrence of depression and anxiety symptoms
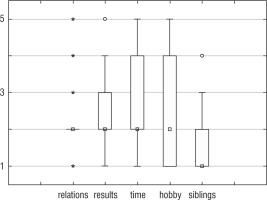
There were no significant differences in the occurrence of symptoms of depression in children measured by children and their parents (for the overall scale score of T-score [p = 0.975], for the emotional problems [p = 0.991] subscale, and the functional problems [p = 0.738] subscale). Statistically significant differences were found between the average assessment of depression signs in girls and boys (Table I). Girls were characterized by a higher level of depression both based on the overall score (p = 0.010) and the emotional problems (p = 0.022) and functional problems (p = 0.012) subscales. Significant differences were found in the parameters assessing anxiety in children and their parents. The distribution of the sten X1 and X2 values of adolescents and parents were different (p = 0.021 and p = 0.001, respectively). There was no significant association between high anxiety levels in adolescents with T1DM and in their parents (p = 0.583, φ = 0.01 for X1 and p = 0.496, φ = 0.04 for X2). No significant correlation was found between diabetes duration time, glycaemic control, the occurrence of acute diabetes complications, and the parameters assessing anxiety and depression (p-value greater than 0.05). In the subjective opinion of the participants, the disease caused the greatest limitation in the sphere of spending free time and pursuing interests. However, even for these variables, less than 30% of the participants believed that the disease was an obstacle in terms of spending free time and developing interests (Figure 1).
Discussion
Depression, anxiety, and stress-related disorders account for significant proportions of mental health problems in T1DM and are associated with less optimal diabetes management, reduced health-related quality of life, increased risk of complications, and premature mortality [2, 19, 20]. According to the literature, about 15% of adolescents with T1DM report elevated levels of psychological distress (depression/anxiety), with potentially negative consequences for self-management [13, 14]. This percentage is similar, for example, to that among patients with asthma (16.5%), but lower than allergies (21.6%), epilepsy (25%), or migraines (43.2%) [21]. For comparison, in the general population, the prevalence of depressive disorders is estimated at 7.5%, and anxiety at about 5% [22, 23]. This may be surprising because, unlike other analysed chronic diseases, the life of a child with T1DM and his/her parents is associated with constant vigilance, analysis of blood glucose measurement results, and the need to independently undertake ongoing interventions, on which the health and life of the child depend.
The period of adolescence and young adulthood is especially difficult for people suffering from chronic diseases. During the pubertal period, most adolescents adapt well to the physiological changes; however, their emotional needs are significantly different from small children and adults [12]. Additionally, during this time, diabetes treatment and self-control are particularly challenging. Apart from only biological changes taking place in the maturing organism, which significantly change, e.g., the daily insulin requirement, a deterioration in glycaemic control is often attributable to inappropriate meal and exercise patterns, poor adherence to treatment regimens, hazardous and risk-taking behaviours, or eating disorders [24–28]. In recent years, significant advances have been made in the therapeutic approaches used in T1DM. The introduction of personal insulin pumps and the development of their technology and glucose monitoring systems seem to be particularly important. On the other hand, therapeutic goals for glycaemic control have become more ambitious. Both of these factors, it seems, can influence the mental and emotional state of adolescents and their parents. Is this a factor affecting treatment outcomes? The analysis of the medical data of the people participating in the study showed that less than a quarter of the studied group achieved the target values of TIR and HbA1c. Interestingly, in contrast to previously published studies, the present study showed a relationship between optimal glycaemic control and a high level of functional problems. According to the literature, the main causes of such difficulties were identified as demands of planning and constant self-control hindering everyday activities, especially those in which peers participate [29]. It also seems to be an important observation of a significant effect of gender on glycaemic control, which was significantly worse in girls. Similar observations were also made in the past. Several studies have found that girls have worse glycaemic control than boys during adolescence [30, 31]. Interestingly, the authors of these studies suggested an association between poorer treatment outcomes in adolescent girls and their higher incidence of depressive disorders. Also in our study group, girls showed higher levels of depression symptoms. However, it was not possible to confirm a direct relationship between these phenomena, and the fact that they were noticed in different groups of respondents indicates the need to pay special attention to the mental state of teenage girls with T1DM. In a recently published large analysis of Swedish registry data, attention was drawn to the psychological burden of families of people who were diagnosed with DM1 as children [2]. The present study, although on a much smaller scale, analysed the occurrence of symptoms of depression and anxiety in both children with diabetes with T1DM and their parents. While the symptoms typical of depression were consistent in children and adults, very interesting results were obtained by analysing the symptoms of anxiety. The level of anxiety in parents was significantly higher than in children. Similar observations were made by researchers analysing the fear of hypoglycaemia in children with T1DM and their parents [32]. Many parents have psychological problems after the diagnosis of T1DM in their children. It has been indicated that approximately 34% of parents report distress at diagnosis, and 19% of parents report distress from 1 to 4 years after diagnosis [33].
Interestingly, in the studied group, we found no significant correlation between the level of anxiety and glycaemic control or the occurrence of hospitalization due to acute complications of T1DM. Contrary to the studies of other authors, who indicated a longer duration of diabetes as a factor positively influencing the quality of life and emotional state of children with diabetes, in the analysed group there was no significant correlation between diabetes duration time, and the parameters assessing anxiety and depression [34].
In the subjective opinion of the participants, the disease caused the greatest limitation in the sphere of spending free time and pursuing interests. This is a particularly important observation because healthcare professionals often focus on medical parameters. It turns out that, regardless of the achieved treatment results and the use of modern therapeutic technologies, T1DM is still a factor that limits the daily functioning of young people.
The strength of the study is that it is an ethnically homogeneous group, living in one geographical area, with similar socio-economic conditions. Simultaneous analysis of the mental state of children and parents is also important. There is also value in the combined analysis of psychological and medical data. The study has some limitations. First, as a study conducted in one centre, in a group of subjects of equal age, it included a relatively small number of participants. Another limitation is the lack of detailed analysis of the social and economic situation, parents’ education, and the use, or not, of the most modern methods of therapy, e.g. advanced hybrid closed-loop.

 ENGLISH
ENGLISH