Plasma cell neoplasms (PCN) represents a group of tumours originating from abnormal plasma cells, which are further classified into multiple myeloma (MM), medullary plasmacytoma (MP), and extramedullary plasmacytoma (EMP) [1, 2]. Extramedullary plasmacytoma is a rare entity and usually involves the nasopharynx or upper respiratory tract [3]. Involvement of the gastrointestinal tract (GIT) occurs in approximately 10% of EMP cases, with the stomach and small intestine being most commonly involved [1, 3–5]. Solitary EMP of the rectum is exceedingly rare [3].
We present a case of an 88-year-old male patient admitted to our clinic for signs and symptoms suggestive of ulcerative colitis relapse. He was diagnosed with ulcerative proctosigmoiditis 5 years earlier and treated with mesalazine. He reported bloody diarrhoea (approximately) 10 bowel movements/day, faecal incontinence, abdominal and back pain, weakness, and weight loss. Examination revealed cachexia, tachycardia, and abdominal tenderness. The patient’s comorbidities included arterial hypertension, chronic obstructive pulmonary disease (COPD), type 2 diabetes, and gallstones. His family history was negative for haematological or gastrointestinal neoplasms. Previous colonoscopy was performed 3 years earlier, showing endoscopic and histological remission. On admission, laboratory analysis revealed microcytic anaemia, mild hypoproteinaemia and hypoalbuminaemia, elevated parameters of inflammation (C-reactive protein 83 mg/l, erythrocyte sedimentation rate 38 mm/h), and elevated faecal calprotectin level (1395 µg/g). Plain chest radiography showed signs of COPD, and plain abdominal X-ray was normal. Stool was negative for Clostridium difficile toxin and cytomegalovirus. Upon admission the patient was started on topical and oral mesalazine therapy, antibiotics, and supportive care.
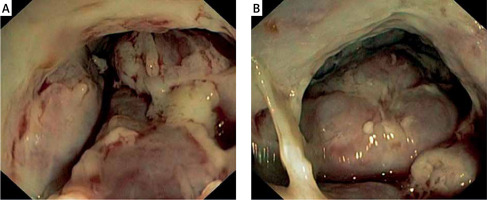
Colonoscopy was performed, revealing tumour-like, whitish, and hard (on biopsies) mass located in the proximal part of the anal canal, rectum, and rectosigmoid junction (Figure 1). Standard histopathology examination was suggestive of immunoproliferative disease, requiring further evaluation. Immunohistochemistry was performed and showed CD38+, κ+, λ–, MUM-1+, EBV-LMP-, CD20-, CD138-, and Ki 67+ in 60% positive cells, indicating plasmablastic plasmacytoma (Figure 2). Further diagnostic algorithm excluded possible differential entities such as myeloma and lymphoma. Serum protein electrophoresis detected paraprotein, and immunofixation identified immunoglobulin G (IgG), λ type. Urine protein electrophoresis did not indicate the presence of Bence-Jones protein. Alkaline phosphatase, calcium level, and lactate dehydrogenase were normal, and the concentration of β2 microglobulin was elevated (13.2 mg/l, reference range: 0.9–3.0 mg/l). Sternal biopsy showed unspecific reactive bone marrow changes without elements of lymphoproliferative disorders. Multislice computed tomography (MSCT) showed a tumour mass 10 × 11 × 16 cm in size occupying the abdomen and pelvis, surrounding the rectum, iun the proximal part of anal canal, with parailiac adenopathy, ascites, and peritoneal involvement, and invading the urinary bladder and prostate (Figure 3). We additionally reviewed slides from previous biopsies, which surprisingly revealed monoclonality despite gross histopathology being normal. His performance status prevented him from receiving further oncological therapy. His condition progressively worsened, and he died 1 month after the diagnosis was established.
Figure 2
High magnification reveals that most of the neoplastic cells are plasmablasts with a prominent eosinophilic nucleolus (A). Immunohistochemistry for the specimens revealed Kappa+ (B), MUM1+ (C), and CD38+ (D). H&E, 100×
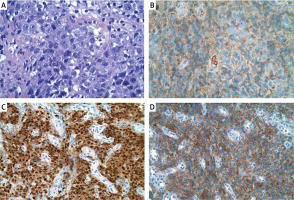
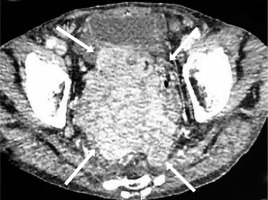
Figure 3
Multislice computed tomography axial scan of the abdomen revealed a tumour mass (arrows) 10 × 11 × 16 cm in size surrounding the rectum and the proximal part of anal canal with peritoneal involvement, invading the urinary bladder and prostate
The cumulative incidence of solitary plasmacytoma was reported as 0.15/100,000 [6]. Diagnosis of SP is made by histological and immunohistochemical analysis of tissue biopsy, and its main characteristic is the presence of a homogenous infiltrate of monoclonal plasma cells positive for CD138 and/or CD38 [7]. Alexiou et al. reported that in 82.2% of cases EMP affected the nasopharynx and upper respiratory tract, which is in line with the result of 80% in the study by Galieni et al. [8]. The occurrence of extramedullary plasmacytoma in the rectum and anal canal is extremely rare. Literature regarding this rare condition is lacking and mostly based on clinical reports. To our knowledge there are only 2 cases of EMP in the rectum and anal canal reported in patients with ulcerative colitis [9, 10].
Presenting symptoms are usually related to the site of plasma cell infiltration. In our case the patient claimed diarrhoea and blood in stool as well as faecal incontinence for the past 2 months. The endoscopic appearance of EMP arising from the gastrointestinal tract may vary, ranging from small sized polyp, as in the case reported previously by Miwa et al., to ulcerated masses or massive, whitish infiltration (as in the present case). Colonoscopy showed a massive tumour, which could be mistaken for adenocarcinoma or a neuroendocrine tumour (NET). Diagnosis of EMP must be supported by biopsy and immunohistochemistry following the exclusion of other possible diseases.
Our patient was treated with mesalazine only, and he had not taken any immunomodulatory therapy; thus, the possibility of these drugs playing a potential role in the development of EMP could not be considered in our case. Lee et al. reported a similar case of EMP presenting with abdominal pain and diarrhoea, but without previously diagnosed ulcerative colitis [1]. Given its clinical presentation, our patient could easily be mistaken for disease relapse. Additionally, it seems that EMP was developing in parallel to UC, but it is not clear whether it was initiated or worsened by chronic inflammation. Given the complexity, a multidisciplinary approach is needed in order to establish a correct diagnosis and therapeutic modality.
Treatment of EMPs varies – from surgery or chemotherapy to radiotherapy (RT) [11]. PCNs are highly sensitive to radiation, confirming that radiation therapy is the standard treatment for solitary bone plasmacytoma and EMP [12, 13]. For patients with EMP, surgery might be able to resect large and well-defined masses but should be followed by radiotherapy [7]. On the other hand, it is not clear whether this would be the treatment of choice in the case of our patient due to an unfavourable event. His performance status prevented him from receiving further oncological therapy. His condition progressively worsened, and he died 1 month after the diagnosis was established.
EMP in patients with UC is extremely rare. Its existence could be sui generis or perhaps complicating a long-standing history of inflammatory bowel disease. Nevertheless, clinicians should be aware of this rare entity even in patients with confirmed previous diagnosis affecting the anorectum (e.g. ulcerative colitis).