Epidemiology, causes, and symptoms of gastrointestinal bleeding
Gastrointestinal bleeding is an acute condition in gastroenterology, frequently prompting urgent hospital admission and increasing both morbidity and mortality, particularly among older patients. Depending on the anatomical location, gastrointestinal bleeding is classified as upper gastrointestinal bleeding (UGIB; 80% of all cases), when the source is located above the duodenojejunal flexure (the ligament of Treitz), or lower gastrointestinal bleeding (LGIB), when the source is located below this ligament. Given the distinct aetiology, treatment modalities, and prognostic factors, UGIB is further subdivided into non-variceal (the most common) and variceal (arising from oesophageal or gastric varices, accounting for 11% of UGIB). Among non-variceal causes, peptic ulcer disease is predominant (duodenal ulcer 28–59%, gastric ulcer 11–24%), followed by erosive (haemorrhagic) inflammation of the oesophageal, gastric, and duodenal mucosa (1–47%), Mallory-Weiss syndrome (4–7%), malignancies (2–4%), and other causes such as vascular lesions in the gastrointestinal tract, haemobilia, pancreatic duct bleeding, and iatrogenic causes (2–7%) [1, 2]. The incidence of UGIB ranges from 40 to 150 cases per 100,000 persons per year and has declined in recent years [3]. However, its mortality rate remains at 2–10% [4]. The most common causes of LGIB include diverticular disease of the colon (20%), anorectal diseases such as haemorrhoids, anal fissures, and rectal ulcers (12–21%), colitis (inflammatory bowel disease, ischaemic colitis), radiation proctitis, iatrogenic factors (e.g., post-polypectomy), angiodysplasia, and colorectal cancer [5]. It should be emphasised that in approximately 23% of LGIB cases, the source of bleeding cannot be identified [6]. The incidence of LGIB is estimated at 33 to 87 cases per 100,000 persons per year [7]. The mortality rate is 0.5–8% but may rise to 18% if bleeding occurs during hospitalisation [6, 8].
Clinical manifestations of gastrointestinal bleeding can be divided into general signs of anaemia (regardless of the bleeding source) and those linked to the bleeding site. General symptoms include weakness, dizziness, syncope, excessive sweating, tachycardia, hypotension, hypovolaemic shock, and exacerbation of chronic coronary artery disease. Haematemesis or coffee-ground vomiting suggests a bleeding source above the ligament of Treitz. In cases of melaena, 90% originate from the upper gastrointestinal tract. Conversely, haematochezia is most commonly the result of intestinal bleeding, although massive upper gastrointestinal haemorrhage – particularly from oesophageal varices – can also present with the severe haematochezia, often alongside haemodynamic instability.
Risk of gastrointestinal bleeding in patients receiving oral anticoagulants
Anticoagulant agents – encompassing vitamin K antagonists (VKAs, e.g. warfarin and acenocumarol), direct (non-vitamin K antagonist) oral anticoagulants (DOACs or NOACs), and antiplatelet drugs (P2Y12 receptor inhibitors such as clopidogrel, prasugrel, and ticagrelor, as well as acetylsalicylic acid [ASA]) – are used in the treatment of patients with atrial fibrillation, ischaemic heart disease, venous thromboembolism, and valvular heart disease. Owing to their high therapeutic efficacy, favourable safety profile, and the lack of a requirement for routine dose monitoring, DOACs have largely replaced VKAs in anticoagulant therapy in recent years. The DOAC group includes one direct thrombin inhibitor (dabigatran) and several factor Xa (FXa) inhibitors (the “xabans”: rivaroxaban, apixaban, edoxaban). An important adverse consequence of oral anticoagulant therapy is an increased risk of gastrointestinal (GI) bleeding. A meta-analysis revealed that VKAs increased the risk of GI bleeding more than threefold compared to placebo and almost doubled it compared to aspirin [9]. Although DOACs, relative to VKAs, are associated with a lower risk of severe bleeding, fatal bleeding, and clinically relevant non-major bleeding, some studies indicate a higher risk of GI bleeding with DOAC use. This observation has significant clinical implications, given that approximately 487,000 people in Poland are currently treated with FXa inhibitors, with numbers expected to rise in the coming years. Available literature data indicate that the annual risk of serious bleeding in patients treated with FXa inhibitors is about 1–4%, and appears higher among those taking rivaroxaban (3–4%) than among those taking apixaban or edoxaban (1.5–2.5%) [10]. In patients receiving FXa inhibitors, haemorrhages most frequently occur in the GI tract (48%), intracranially (17%), or as a result of trauma (26%) [11]. Unlike VKAs, which are prodrugs activated after absorption, orally administered DOACs are active immediately. Any unabsorbed drug can directly affect the GI mucosa, potentially increasing the risk of bleeding. The oral bioavailability of dabigatran is only about 6%, whereas that of FXa inhibitors ranges from 60 to 80% [12]. In the RE-LY trial, the risk of major GI bleeding with dabigatran was dose-dependent and only rose at the 150 mg dose when compared to warfarin (1.51% vs. 1.02% per year; RR 1.50) [13]. Other studies have shown that higher-dose edoxaban (60 mg) was associated with increased GI bleeding compared to warfarin (1.51% vs. 1.23% per year; hazard ratio (HR) 1.23), as was rivaroxaban at 20 mg (2.00% vs. 1.24% per year; HR = 1.66) [14, 15]. In contrast, the ARISTOTLE trial found no significant difference in the rate of major GI bleeding between apixaban (5 mg twice daily) and warfarin (0.76% vs. 0.86% per year; HR = 0.89) [16]. A meta-analysis of 43 randomised clinical trials involving a total of 166,289 patients revealed no difference between DOACs and traditional anticoagulants in terms of the risk of severe GI bleeding (1.5% vs. 1.3%; odds ratio (OR) = 0.98), clinically relevant non-major bleeding (0.6% vs. 0.6%; OR = 0.93), upper GI bleeding (1.5% vs. 1.6%; OR = 0.96), or lower GI bleeding (1.0% vs. 1.0%; OR = 0.88) [17]. However, more detailed analyses of individual DOACs showed that compared to traditional anticoagulants, patients receiving dabigatran had a 23% higher risk of bleeding, while rivaroxaban users had a 40% higher risk. These associations were not observed for apixaban or edoxaban.
In light of these findings, stratifying patients who require long-term anticoagulation according to their risk of GI bleeding is warranted. Impaired renal and hepatic function, advanced age, low body weight, and concomitant antiplatelet therapy all increase the risk of GI bleeding in individuals taking dabigatran or rivaroxaban. Factors associated with higher mortality in patients on oral anticoagulants include older age (> 75 years), intestinal ischaemia, multiple comorbidities, the need for blood transfusion, and LGIB occurring during hospitalisation [18]. Among the predictive scales available for estimating bleeding risk in anticoagulated patients, the HAS-BLED score is particularly effective, incorporating the following risk factors: hypertension, abnormal renal and/or liver function, history of stroke or thromboembolism, history of bleeding or bleeding disorders, age > 65 years, use of aspirin or non-steroidal anti-inflammatory drugs, and alcohol abuse [19].
Management of gastrointestinal bleeding in patients receiving DOAC therapy
Initial management of a patient with GI bleeding who is on DOAC therapy involves taking a detailed history regarding the type, dose, and timing of the last DOAC intake, the use of other medications, comorbidities, any prior bleeding episodes, and an assessment of the patient’s haemodynamic status – considering both the severity and potential source of bleeding. According to the definition proposed by the International Society on Thrombosis and Haemostasis (ISTH), major bleeding is defined as any bleeding that:
leads to death and/or
is symptomatic and occurs in a critical organ or anatomical area and/or
results in a drop in haemoglobin concentration of ≥ 2 g/dl (or to an absolute level ≤ 8 g/dl) or requires the transfusion of ≥ 2 units of whole blood or packed red blood cells [20].
Physical examination should focus on vital signs – blood pressure, heart rate, respiratory rate, oxygen saturation, body temperature – and potential signs of hypovolaemic shock. Intravenous access should be secured, and in haemodynamically unstable patients, fluid resuscitation should be promptly initiated to restore tissue perfusion and prevent multiorgan failure. According to the European Society of Gastrointestinal Endoscopy (ESGE) guidelines, a restrictive use of crystalloids (0.9% NaCl or Ringer’s lactate), possibly combined with inotropic support (e.g. dopamine), leads to fewer adverse events than an aggressive fluid resuscitation strategy [21]. A similarly restrictive approach is recommended for blood transfusions. In haemodynamically stable patients with UGIB or LGIB, who have no history of cardiovascular disease, blood transfusions are advised at a haemoglobin level of ≤ 7 g/dl (with a target post-transfusion haemoglobin level of 7–9 g/dl) [5, 21]. Such management lowers mortality and reduces the risk of rebleeding. In patients with active bleeding and a history of cardiovascular disease, transfusions are recommended at a haemoglobin level of ≤ 8 g/dl (with a target post-transfusion haemoglobin level of ≥ 10 g/dl).
Risk stratification in patients presenting with acute GI bleeding can aid in selecting those who require hospital admission or not. The Glasgow-Blatchford score is used to estimate the risk associated with UGIB before endoscopy (Table I) [22]. Patients scoring ≤ 1 on the Glasgow-Blatchford scale have a very low risk of rebleeding, 30-day mortality, or need for in-hospital intervention; they can safely undergo outpatient gastroscopy. For LGIB, the Oakland score (Table II) can help determine whether hospital admission is necessary [23]. In patients with self-limiting bleeding, no unfavourable clinical factors, and an Oakland score ≤ 8, the ESGE recommends outpatient diagnostic evaluation for the cause of LGIB [5].
Table I
Glasgow-Blatchford Score (adapted from: Blatchford et al.)
Table II
Oakland Score (adapted from: Oakland et al.)
In initial management of non-variceal UGIB, an intravenous high-dose proton pump inhibitor (PPI) reduces the need for therapeutic intervention during panendoscopy. A PPI bolus (equivalent to 80 mg omeprazole) should be administered, followed by a continuous infusion of 8 mg/h for up to 72 h, and then switched to oral PPI therapy. In patients with liver cirrhosis and variceal bleeding, terlipressin, somatostatin or its analogues (which reduce splanchnic blood flow) and antibiotic prophylaxis (which lowers infection risk and mortality) should be administered.
In patients receiving DOACs who present with GI bleeding, it may be necessary to withhold or reverse anticoagulant therapy. Ideally, this decision – made in consultation with a cardiologist or haematologist – should balance the bleeding risk associated with DOACs against the thromboembolic risks of discontinuing therapy. A study comparing patients with GI bleeding (46.8% UGIB and 53.2% LGIB) on oral anticoagulants (DOACs or vitamin K antagonists [VKAs]) versus those not receiving anticoagulants found no difference between the groups in the need for endoscopic therapy or blood transfusion, rebleeding rates, or thromboembolic complications [24]. Furthermore, correction of INR, reversal of anticoagulant effect, and discontinuation of anticoagulants were associated with a higher risk of thromboembolic events. These data suggest that early endoscopic intervention in acute GI bleeding may be performed without modifying anticoagulant therapy. ESGE guidelines recommend temporarily withholding DOACs in patients with active UGIB, but this should not delay gastroscopy or endoscopic treatment [21]. Similar guidance applies to clinically significant LGIB [5, 7]. However, in patients with minor, self-limiting LGIB (Oakland score ≤ 8), ongoing DOAC therapy may be continued. These recommendations stem from the pharmacodynamic profile of DOACs. On the one hand, they have a rapid onset of action (achieving full anticoagulant effect within about 3 h of dosing); on the other, they have relatively short half-lives (8–12 h), meaning their effect typically resolves within 24 h. Consequently, details about the dose and timing of the last administered dose are crucial. Interpreting the anticoagulant effect of DOACs using standard laboratory parameters – such as the prothrombin time (PT) and activated partial thromboplastin time (aPTT) – may be difficult, as normal values do not rule out clinically significant plasma concentrations of apixaban or rivaroxaban. If available, measuring anti-FXa activity may be helpful.
In most patients with LGIB, temporarily discontinuing the anticoagulant and providing fluid resuscitation while awaiting the cessation of the drug’s anticoagulant effect is sufficient. In patients with renal impairment, the duration of action of DOACs – particularly dabigatran – may be prolonged. Haemodialysis has been shown to effectively remove dabigatran from the plasma and prevent rebleeding [25]. If up to 3–4 h have elapsed since the last DOAC dose, administration of activated charcoal may be considered. Vitamin K, fresh frozen plasma (FFP), and protamine sulphate are ineffective in patients treated with DOACs. Given the lack of robust scientific data, a joint position paper by the American College of Gastroenterology (ACG) and the Canadian Association of Gastroenterology (CAG) does not recommend the routine use of prothrombin complex concentrate (PCC) in DOAC-treated patients with gastrointestinal bleeding [26]. PCC administration should only be considered in a selected group of patients with life-threatening GI bleeding who have received a DOAC dose within the past 24 h. ESGE guidelines similarly endorse using PCC exclusively for severe, ongoing upper (UGIB) and lower (LGIB) gastrointestinal bleeding [5, 21]. In a systematic review of 10 studies (case series, total of 340 patients) assessing the efficacy of PCC in reversing the effect of FXa inhibitors in patients with severe bleeding, the proportion of patients achieving effective haemostasis was 69% [27]. Based on this analysis, it remains unclear whether PCC administration combined with DOAC discontinuation is more effective than simply withholding the drug in patients with severe bleeding related to an FXa inhibitor.
According to ESGE guidelines, following initial resuscitation in non-variceal UGIB, endoscopy should be performed within 24 hours [21]. Such a strategy has been shown to reduce in-hospital mortality, shorten hospital stays, and lower treatment costs. In a selected group of patients with severe or persistent active bleeding, prokinetic agents are recommended to increase the likelihood of gastric emptying. A single intravenous dose of erythromycin (250 mg administered 30–120 min before endoscopy) has been shown to enhance visualisation of the gastric mucosa, reduce the need for repeat endoscopy, and shorten hospital stay [28]. In cases with an elevated risk of gastric content aspiration into the respiratory tract (ongoing haematemesis, encephalopathy, or severe agitation), endotracheal intubation is advised. The decision to use endoscopic haemostatic methods depends on the endoscopy findings, typically based on the Forrest classification (Table III) [29]. Endoscopic therapy is indicated in active bleeding (Forrest Ia and Ib) and in lesions showing a non-bleeding visible vessel (Forrest IIa). If a lesion classified as Forrest IIb (adherent clot) is identified, removal of the clot should be considered, and endoscopic therapy instituted if active bleeding or a visible vessel is discovered. Forrest IIc and III lesions do not require endoscopic intervention. The optimal method for endoscopic treatment of active ulcer bleeding (Forrest Ia or Ib) is a combination of epinephrine injection plus a thermal, mechanical, or sclerosant injection therapy [21]. In the case of a visible vessel (Forrest IIa), thermal, mechanical, or sclerosant therapy – alone or in combination with an epinephrine injection – is recommended. If a clot is present in the ulcer base, it should be removed after dilute epinephrine injection, followed by endoscopic therapy based on high-risk features. In patients with persistent active bleeding despite standard haemostatic techniques, ESGE suggests the use of over-the-scope clips (OTSC) or haemostatic powder [21]. In situations where rebleeding occurs and endoscopic haemostasis cannot be achieved, transcatheter angiographic embolisation (TAE) or surgical treatment should be considered.
Table III
Forrest Score of endoscopic signs of upper GI bleeding (adapted from: Forrest et al.)
For LGIB, in haemodynamically stable patients, the recommended first-line investigation is elective colonoscopy during hospitalisation, following adequate bowel preparation with 4–6 l of polyethylene glycol solution [5]. Colonoscopy allows for simultaneous diagnosis, biopsy if necessary, and endoscopic haemostasis. By contrast, in haemodynamically unstable patients with suspected active LGIB, computed tomography angiography (angio-CT) should be performed before colonoscopy. Notably, in 8–9% of patients presenting with LGIB symptoms, the bleeding source is located in the upper GI tract [5]. Therefore, according to the British Society of Gastroenterology (BSG) guidelines, if angio-CT in a patient in shock does not identify the bleeding source, panendoscopy should be performed first [7]. The choice of the optimal haemostatic method depends on the underlying cause of LGIB. Radiological embolisation should be considered for haemodynamically unstable patients in whom angio-CT identifies a bleeding site or in whom endoscopic haemostasis is unsuccessful. In exceptional situations where both endoscopic and radiological methods fail, surgical intervention remains an option.
The decision to resume anticoagulant therapy should account for the rapid onset of DOAC action, the patient’s risk of thrombosis, the risk of rebleeding, patient preferences, and input from the cardiologist or haematologist. European and North American gastroenterology societies concur that oral anticoagulants should be restarted immediately once bleeding is controlled, ideally within 7 days, while considering each patient’s thromboembolic risk and recognising the considerably faster onset of DOAC action compared to VKAs.
Subsequent management of patients on DOACs with ongoing GI bleeding should be tailored to the severity of bleeding, the clinical condition, and patient risk factors. In cases without improvement in patients with life-threatening or critical-organ bleeding, specific reversal of anticoagulant effects is indicated. Current recommendations from multiple professional societies, also in the field of gastroenterology, advise administering idarucizumab (5 g IV) to dabigatran-treated patients, or andexanet alfa to patients treated with rivaroxaban or apixaban [11, 21, 30–33]. In the absence of a suitable antidote, PCC or activated PCC (aPCC) may be considered for delayed reversal of DOAC effects.
Characteristics and efficacy of andexanet alfa
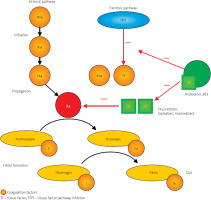
Andexanet alfa (AA) is an inactive analogue of human coagulation factor Xa (FXa) that effectively reverses the activity of FXa inhibitors, namely rivaroxaban and apixaban. AA is a recombinant form of human FXa in which the serine residue in the enzyme’s active site has been replaced by alanine [30, 34, 35], and the gamma-carboxyglutamic acid domain has been removed. As a result of these modifications, AA is unable to cleave or activate prothrombin and does not bind to the prothrombinase complex. It specifically binds to and sequesters FXa inhibitors (apixaban and rivaroxaban), thereby reversing their anticoagulant effect. FXa inhibitors function by blocking activated factor Xa in the coagulation cascade, leading to reduced thrombin generation and, consequently, a lower risk of thrombus formation in veins and arteries. Moreover, AA binds to the tissue factor pathway inhibitor, attenuating its activity and potentially increasing thrombin production via the tissue factor pathway, resulting in a transient procoagulant effect [30, 34]; see Figure 1.
Andexanet alfa represents a therapeutic option with efficacy and safety confirmed by clinical trials. It is the only specific agent used to reverse anticoagulation induced by FXa inhibitors in patients with life-threatening or uncontrolled haemorrhages, including gastrointestinal bleeding. In November 2013, it was granted breakthrough therapy status [36], and in May 2018, the United States Food and Drug Administration (FDA) approved it using the accelerated approval program [37]. Subsequently, in April 2019, the European Medicines Agency (EMA) [38] granted AA a conditional marketing authorisation which is used for medicinal products of particular importance to public health. Under this conditional marketing authorisation, the EMA requires ongoing post-marketing studies to confirm efficacy of the treatment.
The main evidence underpinning the approval of andexanet alfa comes from the ANNEXA trial programme. In two phase III studies, healthy volunteers received apixaban (ANNEXA-A) or rivaroxaban (ANNEXA-R), followed by AA to reverse the effect of these FXa inhibitors. These studies showed a rapid reduction in anti-FXa activity after administration of AA – 94% for apixaban and 92% for rivaroxaban – without any severe adverse events [39]. A major advantage of AA is the speed of onset of action following intravenous administration. The aforementioned clinical trials confirmed that the drug reduced FXa inhibitor activity within 2–5 min of administration and subsequently restored normal haemostasis [39]. A subsequent phase III/IV clinical study, ANNEXA-4, evaluated andexanet alfa in 479 patients with life-threatening or uncontrolled bleeding, receiving anticoagulation with apixaban (51%), rivaroxaban (37%), edoxaban (8%), or enoxaparin (5%) [35]. Most haemorrhages were intracranial (69%) or gastrointestinal (22%). Anti-FXa activity decreased by 93% and 94% after AA administration in patients treated with apixaban and rivaroxaban, respectively. Haemostasis was assessed as good or excellent in 81% of patients on rivaroxaban and 79% of those on apixaban. Treatment efficacy was independent of age, sex, the specific FXa inhibitor used, and the site of bleeding [35]. A thromboembolic event within 30 days of AA administration occurred in 10% of patients, with only one serious infusion-related reaction reported. Notably, no thromboembolic events were observed in patients who resumed FXa inhibitor therapy early. A total of 75 deaths (16%) were recorded within 30 days of the bleeding event, with the highest mortality (17%) in patients who had experienced intracranial bleeding. Mortality rates were slightly lower among patients with gastrointestinal bleeding (12%) and in those with other bleeding sites (15%) [35]. Observational studies and indirect comparisons also confirm the high efficacy of AA and its superiority over non-specific strategies for treating life-threatening haemorrhages.
Real-world evidence supports the excellent haemostatic efficacy of andexanet alfa, especially in patients with intracranial haemorrhage treated early [40–43]. These clinical data also show that, compared with non-specific FXa inhibitor reversal strategies, AA increases hospital survival rates irrespective of the bleeding site. In a real-world evidence (RWE) study by Sutton et al., in-hospital mortality following AA administration was threefold lower than in patients who received 4-factor prothrombin complex concentrate for bleeding management (10.6% vs. 25.3%; OR = 0.31), and thirty-day mortality was reduced by nearly a half in the andexanet alfa group (20.0% vs. 32.4%; OR = 0.54) [44]. In another clinical study, Dobesh et al. found that the risk of in-hospital death for patients with bleeding at any site was reduced by 50% in those treated with AA compared with those receiving 4-factor prothrombin complex concentrate [45]. Indirect comparisons based on the ANNEXA-4 study and earlier studies for PCC and the standard of care also indicate the superiority of andexanet alfa in reducing 30-day mortality, achieving effective hemostasis, and reducing the progression of hematoma [46]. These analyses suggest particularly marked mortality risk reductions (up to 69%) in patients with intracranial bleeding.
Andexanet alfa is recommended by major scientific and clinical societies in various fields (Table IV) [26, 47–49].
Table IV
Scientific societies’ recommendations for using andexanet alfa
The studies presented above indicate that andexanet alfa is a highly effective therapeutic option for reversing the anticoagulant effects of FXa inhibitors. Owing to its properties, AA is now an important treatment component suggested in the latest recommendations from European and American gastroenterological societies for life-threatening gastrointestinal (GI) haemorrhages:
2021 – Guidelines of the European Society of Gastrointestinal Endoscopy (ESGE) and the British Society of Gastroenterology (BSG) recommend using AA to reverse the activity of FXa inhibitors only in bleeding patients with haemodynamic instability
(weak recommendation; low quality of evidence) [50].
2021 – Guidelines of the European Society of Gastrointestinal Endoscopy (ESGE) for Non-Variceal Upper Gastrointestinal Bleeding advise temporary discontinuation of anticoagulants, including FXa inhibitors; however, this approach should not delay panendoscopic evaluation, and in cases of severe, ongoing bleeding, reversal agents should be considered
(strong recommendation; low quality of evidence) [21].
2022 – Guidelines of the European Society of Gastrointestinal Endoscopy (ESGE) for Variceal Upper Gastrointestinal Bleeding recommend using reversal agents for FXa inhibitors only if haemodynamic stability cannot be achieved, and any clinical decision to administer AA should be made in collaboration with a haematologist, taking into account the thromboembolic risk associated with this agent
(strong recommendation; low quality of evidence) [51].
2021 – Guidelines of the European Society of Gastrointestinal Endoscopy (ESGE) for Lower Gastrointestinal Bleeding suggest AA for ongoing life-threatening bleeding that persists despite endoscopic therapy and continued haemodynamic instability
(weak recommendation; low quality of evidence) [5].
2022 – American College of Gastroenterology (ACG) and Canadian Association of Gastroenterology (CAG) Guidelines on Upper Gastrointestinal Bleeding do not recommend the use of AA in patients with suspected FXa inhibitor-related GI bleeding (conditional recommendation; very low quality of evidence); this position is based on the very low quality of existing literature evidence (including lack of a control group and methodological inconsistencies in endoscopic management), high costs, and potential adverse effects of andexanet alfa. These guidelines do allow for AA use in life-threatening GI haemorrhage in patients who have taken/took rivaroxaban or apixaban within the previous 24 h [26].
2023 – American College of Gastroenterology (ACG) Guidelines on Lower Gastrointestinal Bleeding recommend using DOAC reversal agents in a small subset of patients with ongoing life-threatening bleeding that has not improved after drug discontinuation and fluid resuscitation; the drugs (idarucizumab for patients taking dabigatran and AA for those on apixaban or rivaroxaban) may be administered if the direct oral anticoagulant was taken within the preceding 24 hours (conditional recommendation; very low quality of evidence) [33].
In summary, across the above guidelines addressing GI bleeding associated with FXa inhibitors, andexanet alfa administration is reserved for patients with severe, life-threatening haemorrhage – including GI bleeding – where there is no improvement after drug discontinuation and initial therapy.
Andexanet alfa dosing regimen
Administration of a specific FXa inhibitor reversal agent should follow the product’s prescribing information. For AA, the dose depends on the amount of rivaroxaban or apixaban taken and the time since the last dose (Table V).
Table V
Dosage of andexanet alfa for dose reversal of apixaban or rivaroxaban
| Inhibitor | Last dose | Time of the last dose before starting andexanet alfa | |
|---|---|---|---|
| < 8 hours | ≥ 8 hours | ||
| Apixaban | ≤ 5 mg | Low dose | Low dose |
| > 5 mg | High dose | ||
| Rivaroxaban | ≤ 10 mg | Low dose | Low dose |
| 10 mg | High dose | ||
AA is given as an intravenous bolus at a rate of 30 mg/min over 15 min (low dose: 400 mg) or 30 min (high dose: 800 mg), followed by a continuous infusion of 4 mg/min (low dose: 480 mg) or 8 mg/min (high dose: 960 mg) over 120 min (Table VI). Maximum reversal of anti-FXa activity occurs within 2 min of completing the bolus. Continued administration of the drug in an intravenous infusion maintains a reduction in anti-FXa activity for up to 3 hours after the end of the infusion. At that point, anti-FXa concentrations return to the placebo level or above [38, 39, 52, 53]. A clinical decision to initiate treatment with andexanet alfa should ideally take into account the baseline anti-FXa activity level, provided this measurement is available in a timely manner.
Safety of andexanet alfa
The most frequently observed adverse reactions associated with AA administration are mild infusion-related events, such as a sensation of warmth, facial flushing, excessive sweating, and chest discomfort. In cases of mild symptoms, careful patient monitoring is sufficient, whereas more pronounced symptoms may require a slower infusion rate or a brief interruption, alongside consideration of an antihistamine. It is important to remember that AA also exerts an independent procoagulant effect, attributable to inhibition of tissue factor pathway inhibitor (TFPI) [54, 55]. The duration of the elevated thrombotic risk in patients who received AA is unknown, but thromboembolic events may occur up to 30 days after administration. This increased risk is likely multifactorial, involving the use of AA as an antidote, but also the discontinuation of anticoagulation, hypercoagulability induced by bleeding itself, and patient immobility during hospitalisation [52, 54, 55]. No thromboembolic events were reported in clinical studies of healthy volunteers. In contrast, among patients requiring anticoagulation who experienced severe haemorrhage, thrombotic events occurred in about 10% of cases [39]. In the ANNEXA-4 trial, thromboembolic complications (cerebrovascular events, deep vein thrombosis, pulmonary embolism, myocardial infarction) were observed in 10.4% of patients, with a median onset of 9 days [39]. Notably, no such events occurred in any patient who promptly resumed DOAC therapy. Of the 50 patients who developed thromboembolic complications, 34 had not restarted anticoagulation or shown signs of thrombosis before its resumption. Given the increased thrombotic risk following AA, close patient monitoring is essential for early detection of thrombosis [52]. It is also crucial to restart anticoagulation as soon as clinically feasible following gastrointestinal bleeding. Randomised data on the optimal timing for reintroducing anticoagulants after AA has been used for the emergency management of severe, life-threatening GI bleeding are lacking [52]. Treatment decisions must therefore be made on an individual basis by experienced clinicians, weighing potential benefits against risks [52, 53]. Factors associated with an elevated risk of recurrent GI bleeding include: 1. No identified bleeding source or reversible cause; 2. Bleeding occurring during a DOAC treatment interruption; 3. Multiple angiodysplasia lesions in the GI tract; 4. Advanced age; 5. Alcohol abuse [52]. It should be emphasised that despite the heightened thrombotic risk, the benefit of treating life-threatening bleeding with AA outweighs its associated risks, given that a greater proportion of patients achieve haemostasis compared to the number who develop thrombotic events. AA is characterised by low renal clearance [39, 52]. However, it is rapidly degraded in plasma by endogenous proteases, resulting in a short half-life of approximately 1 h. Contraindications for AA include known hypersensitivity to the active substance or other components of the preparation. Because no clinical data exist on the safety of combining AA with prothrombin complex concentrate, activated prothrombin complex concentrate, recombinant factor VIIa, fresh frozen plasma, or whole blood, such combination therapy should be avoided owing to reports suggesting an increased risk with concurrent use. Monitoring patients receiving AA should focus primarily on clinical assessment. Measuring anti-FXa activity is not recommended for monitoring the reversal of the anticoagulant effect of FXa inhibitors [30, 56].
Expert position of the Polish Society of Gastroenterology
In April 2024, a meeting of experts from the Polish Society of Gastroenterology took place, with the participation of a haematology specialist. After reviewing the available literature, the experts discussed various issues regarding GI bleeding associated with the use of DOACs. They carefully analysed current treatment approaches, including both non-specific and specific reversal strategies – particularly AA for patients with severe, life-threatening gastrointestinal bleeding [48]. In the context of Polish clinical practice, the following core criteria for qualifying a patient with GI bleeding to andexanet alfa therapy were agreed upon:
age ≥ 18 years,
presence of life-threatening or uncontrolled GI bleeding with signs or symptoms of hypovolaemia and haemodynamic instability (e.g. signs of shock with overt bleeding; altered consciousness/mental status; hypotension, i.e. systolic blood pressure < 90 mm Hg; tachycardia > 100 bpm; cold extremities; cyanosis; sweating; pallor; cool and clammy skin; delayed capillary refill),
lack of response to initial fluid resuscitation (pre-endoscopic administration of andexanet alfa), or persistent bleeding despite endoscopic intervention (post-endoscopic administration),
verification that an FXa inhibitor (apixaban or rivaroxaban) was taken within the past 15 h. If a patient took a ‘xaban’ within the last 8 h, it is sufficient to confirm FXa inhibitor use based on medical history. If the timing of FXa inhibitor administration is unknown or > 8 h have passed, measurement of anti-FXa activity is required, provided it can be performed within an acceptable time frame. A diagram illustrating the criteria for patient eligibility and the administration method of andexanet alfa in individuals with severe, life-threatening gastrointestinal bleeding is presented in Figure 2.
Summary
Currently, around 490,000 individuals in Poland undergo anticoagulant therapy with an FXa inhibitor (rivaroxaban or apixaban) each year. Bleeding events associated with oral anticoagulants contribute to increased mortality and disability. As populations age, the incidence of thromboembolic disorders will rise, driving greater demand for anticoagulant prophylaxis and therapy, especially given the growing availability of DOACs (including more affordable generic versions). Consequently, a significant increase in GI bleeding cases – particularly life-threatening haemorrhages – can be expected. Existing therapeutic approaches are insufficient. Until recently, management of life-threatening GI bleeding under FXa inhibitor therapy has relied on symptomatic treatment and non-specific reversal strategies (e.g. prothrombin complex concentrate or recombinant factor VIIa), which lack evidence for efficacy and safety from clinical trials and are used off-label in life-threatening bleeding. Given the need for rapid and effective intervention in life-threatening GI haemorrhage, current management strategies may be inadequate. Introducing a specific therapy (to reverse DOAC effects) could improve treatment outcomes. Andexanet alfa is the first targeted strategy to reverse coagulation for life-threatening GI bleeding related to rivaroxaban or apixaban, offering a rapid onset of action and high efficacy supported by clinical trials.
Before initiating treatment, a complete coagulation panel should be performed, including DOAC level measurement if possible. Chromogenic anti-FXa assays specific to rivaroxaban or apixaban are considered reliable methods to quantify plasma levels [52]. Demonstrating no detectable anti-FXa activity effectively rules out a clinically significant serum DOAC concentration, in which case AA is not indicated. The dose of andexanet depends only on the dose and timing of rivaroxaban or apixaban administration. AA is administered as a bolus followed by a 120-minute intravenous infusion. In Poland, a multidisciplinary expert statement on the use of AA for life-threatening DOAC-associated bleeding was published in 2023. The authors assert that:
Andexanet alfa should be considered for all patients treated with FXa inhibitors who present with life-threatening bleeding and in whom haemostasis cannot be achieved, regardless of the bleeding site.
Andexanet alfa should be available in all stroke centres, trauma centres, and facilities that perform endoscopic management of GI bleeding.
In order to optimise efficacy and minimise the risks associated with andexanet alfa, each of these centres should develop a local protocol for patients receiving oral anticoagulants – or suspected of receiving these drugs – who present with acute, severe bleeding.
Because of the risk of thrombotic complications after administering andexanet alfa, a causal link between FXa inhibitor therapy and the bleeding event should be confirmed by anti-FXa activity assays or direct FXa inhibitor concentration measurements; however, laboratory testing must not delay administration of andexanet alfa [49].
As yet, public funding for AA is not established in Poland. There remains a need to create dedicated reimbursement pathways or identify alternative financing methods for this medication among a small subset of hospitalised patients. Reimbursement for the appropriate use of AA could improve the optimisation of treatment – shortening hospital stays and improving outcomes in patients with severe, life-threatening gastrointestinal bleeding.