Introduction
Acute pancreatitis (AP) is an acute inflammatory disease of the pancreas and is characterised by sudden onset of upper abdominal pain and elevation of serum pancreatic enzymes [1]. The most common causes are excessive alcohol consumption and gallstones. Other rare causes are post endoscopic-retrograde cholangiopancreatography (ERCP), drugs, viral infections, hypertriglyceridemia, hypercalcemia, abdominal trauma, and other idiopathic reasons [2]. About 80% of all AP cases have a mild course and resolve without any complications, only with supportive care. But 20% of all cases develop severe acute pancreatitis, and it is associated with persistent organ failure and a mortality rate of 5–70%. Early diagnosis and accurate prognostic evaluation play an important role in reducing mortality and morbidity [3].
It has been widely accepted that trypsin activation within pancreatic acinar cells is the initiating condition that leads to pancreas autodigestion. As trypsin is activated the attraction and activation of polymorphonuclear leukocytes, macrophages, and lymphocytes starts. They release proinflammatory mediators such as interleukin-1 (IL-1), IL-6, IL-18, tumour necrosis factor α (TNF-α), oxygen free radicals, adhesion molecules, chemokines, and anti-inflammatory cytokines such as IL-11 and IL-10. These substances stimulate the inflammatory process in the gland. If inflammation is not sufficiently restricted by anti-inflammatory cytokines, localised inflammation enhances to systemic inflammation and may lead to tissue damage, organ failure, and death [4, 5].
Little is known about the effects of anti-inflammatory cytokines in AP although much more is known about proinflammatory cytokines. Interleukin-10 is one of the most studied anti-inflammatory cytokines studied in AP [6–8]. But there is a discrepancy among studies in terms of serum IL-10 levels. While in some studies serum IL-10 levels were significantly higher in mild AP than severe AP, it was found to be low in others [6, 7]. In one study it was revealed that serum IL-10 and IL-11 levels signifies the severity of AP. IL-10 and IL-11 have been shown to decrease the severity of AP [6].
Interleukin-35 is a heterodimeric cytokine, which is composed of the Epstein-Barr virus-induced gene 3 (EBI3) and the p35 subunit of IL-12. It is a newly defined potent anti- inflammatory cytokine that is predominantly produced by Foxp3+ regulatory T cells (Tregs). It supresses T-helper (Th) 1, Th2, and Th17 cell responses and plays an important role in host immunity [9]. Interleukin-35 expression is affected in some immune and inflammatory conditions such as systemic sclerosis, asthma and chronic obstructive pulmonary disease (COPD), rheumatoid arthritis, inflammatory bowel disease, and sepsis [9–13].
Acute-phase proteins: TNF-α, IL-6, serum amyloid A (SAA), and more commonly C- reactive protein (CRP) are used in the evaluation of AP patients [14, 15]. Pentraxin-3 is a member of the pentraxin family, and it is a secretory protein classified as a long pentraxin. Both CRP and SAA belong to the short pentraxin subfamily. Pentraxin-3 is expressed by a variety of cells at inflammatory sites and released from neutrophil-specific granules when stimulated with proinflammatory cytokines, bacterial lipopolysaccharides, or by the activation of Toll-like receptors (TLR). Elevated serum pentraxin-3 levels were found in several diseases such as cardiovascular diseases, malignancies, specific infections, and sepsis and correlated with organ dysfunction [16].
Aim
The aim of this study was to evaluate serum pentraxin-3 and IL-35 levels in the early phase of mild AP.
Material and methods
Patients
The study included 83 patients admitted to the Kecioren Training and Research Hospital, Ankara, Turkey between March 2017 and June 2017 with AP symptoms within 36 hours before admission. The diagnosis of AP was based on acute abdominal pain, three-fold increase in pancreatic enzymes (amylase and/or lipase), and abdominal ultrasound. The severity of disease was classified according to the revised Atlanta classification system, and all patients had mild disease. None of the patients had a history of AP and all of them hospitalised for palliative treatment. The time duration between pain onset and hospital admission was as follows: 0–6 h in 12 patients, 6–12 h in 26 patients, 12–24 h in 31 patients, and 24–36 h in 14 patients. Forty-three patients had biliary originated pancreatitis, 2 patients had alcohol-induced pancreatitis, 2 patients had hypertriglyceridemia-related pancreatitis, and 36 patients had idiopathic pancreatitis. There were no AP patients due to drug, metabolic causes and anatomical abnormalities. On evaluation, 24 patients had pancreatic oedema, 2 patients had peripancreatic fluid, 9 patients had pancreatic oedema and peripancreatic fluid, and 2 patients had pancreatic or peripancreatic necrosis (Table I).
Table I
Clinical characteristics of acute pancreatitis patients (n = 83)
The implementation of the study was endorsed by an Ethics Committee (26.04.2017, 2012-kaek-15/1407).
Measurement of serum IL-35 and pentraxin-3 levels
Serum samples were collected at the time of diagnosis and at the 48th h after diagnosis. Within 30 min following collection, the blood was centrifuged (5000 rpm, 5 min) and the serum samples stored at –80°C until analysis. Serum IL-35 concentrations were quantified by using a commercial human IL-35 ELISA kit (Eastbiopharm, China) and human pentraxin-3 ELISA kit (Boster Immunoleader, USA), according to the manufacturers’ protocols. All samples were assayed in duplicate. The mean concentration was determined for each sample.
Statistical analysis
Statistical analyses were performed using the computer program SPSS 22.0 (IBM, USA). Levene’s test of normality was used to test the distribution of variables. Independent sample t-test and paired sample t-test were used for comparison of more than two group means. Mann Whitney-U and Kruskal Wallis tests were used to analyse nonparametric data. The c2 test was used to compare nominal data between AP and control groups. Pearson correlation was used to evaluate the linear relationship between the tested biomarkers. Data were presented as means ± standard deviation or number and percentage. Differences were considered significant at p < 0.05.
Results
Serum IL-35 and penraxin-3 levels in patients with acute pancreatitis
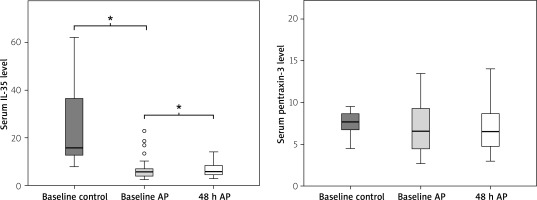
A total of 83 patients with AP and 30 healthy controls were evaluated for serum levels of IL-35 and pentraxin-3. The demographic features of the patients included in the study are summarised in Table II. There were no significant differences between the AP and control group with respect to age, gender, smoking status, hypertension, and body mass index (BMI) (p > 0.05). As shown in Table III, the mean value of the serum IL-35 level in patients with acute pancreatitis at admission was 5.91 ng/ml (4.21–7.90), which was significantly lower than in healthy controls – 25.53 ng/ml (12.79–54.73, p < 0.001). The mean value of serum IL-35 at the 48th h was 6.79 ng/ml (4.42–9.62) and there was a significant difference between that and the admission value (p = 0.015). There was also a significant difference between the serum IL-35 levels at the 48th h in patients with AP and in healthy controls (p < 0.001). The mean value of serum pentraxin-3 levels in patients at the time of admission was 6.75 ng/ml (4.42–9.62) and there was no significant difference between that and healthy controls, 7.64 ng/ml (6.58–8.62, p > 0.05). There was no significant difference between the mean value at admission and the mean value at the 48th h, 6.75 ng/ml (4.74–9.06, p > 0.05) (Figure 1).
Table II
Characteristics of subjects
| Characteristic | Acute pancreatitis (n = 83) N (%) or mean ± SD | Control (n = 30) | P-value* |
|---|---|---|---|
| Age [years] | 52.9 ±15.61 | 47.1 ±13.95 | NS |
| Female sex | 53 (63.9) | 18 (60.0) | NS |
| Smokers | 25 (30.1) | 12 (40.0) | NS |
| BMI [kg/m2] | 26.5 ±3.53 | 25.8 ±3.53 | NS |
| HDL [mg/dl] | 42.3 ±12.44 | 58.7 ±12.39 | < 0.001 |
| LDL [mg/dl] | 106.9 ±36.18 | 86.4 ±18.55 | 0.017 |
| Total cholesterol [mg/dl] | 135.8 ±166.47 | 109.5 ±26.44 | NS |
| Triglycerides [mg/dl] | 172.4 ±54.77 | 158.6 ±33.05 | NS |
Table III
Results of selected laboratory tests at the time of diagnosis and at 48 h from the onset of AP
| Variable | Time point | AP (n = 83) | Control (n = 30) | P-value¥ |
|---|---|---|---|---|
| Amylase [U/l] | Diagnosis 48 h | 592 (292–1200) 80 (61–123)* | 28.5 (24.0–42.0) | < 0.001 |
| Lipase [U/l] | Diagnosis 48 h | 1200 (623–1994) 67 (36–173)* | 24.8 (22.0–32.0) | < 0.001 |
| C-reactive protein [mg/l] | Diagnosis 48 h | 2.02 (0.70–5.46) 2.18 (0.61–7.93)* | 0.26 (0.24–0.34) | NS |
| Sedimentation rate [mm/h] | Diagnosis 48 h | 14.5 (6.0–24.3) 20.0 (12.0–30.0) | 12.0 (10.0–14.0) | 0.012 |
| Leukocytes [× 103/mm3] | Diagnosis 48 h | 9.3 (7.1–11.4) 7.98 (6.26–10.0)* | 7.9 (6.9–8.7) | 0.001 |
| Neutrophils [× 103/mm3] | Fiagnosis 48 h | 7.13 ±3.73 5.41 ±2.77* | 4.74 ±0.94 | 0.005 |
| Lymphocytes [× 103/mm3] | Diagnosis 48 h | 1.78 ±0.88 2.13 ±1.39* | 2.65 ±0.83 | < 0.001 |
| Monocytes [× 103/mm3] | Diagnosis 48 h | 0.65 ±0.24 0.74 ±1.08 | 0.42 ±0.16 | < 0.001 |
| RDW (%) | Diagnosis 48 h | 15.5 (14.8–16.4) 15.4 (14.6–16.5) | 15.6 (15.5–16.0) | NS |
| MPV [fl] | Diagnosis 48 h | 7.82 (7.0–8.9) 7.76 (7.0–9.2) | 10.1 (8.9–10.9) | NS |
| PDW (%) | Diagnosis 48 h | 18.0 (17.2–19.0) 18.1 (17.4–19.0) | 16.8 (16.6–17.2) | NS |
| Platelet count [× 103 μl] | Diagnosis 48 h | 228.6 ±71.98 233.2 ±79.36 | 269.2 ±38.39 | 0.001 |
| Haematocrit (%) | Diagnosis 48 h | 37.9 ±5.24 37.7 ±4.88 | 40.5 ±1.66 | < 0.001 |
| Platelets [× 103/mm3] | Diagnosis 48 h | 0.18 ±0.06 2.29 ±18.64 | 0.24 ±0.04 | < 0.001 |
| Glucose [g/dl] | Diagnosis 48 h | 99.0 (88.0–122.0) 91.0 (82.0–106.5)* | 93.0 (86.0–98.0) | < 0.001 |
| Albumin [g/l] | Diagnosis 48 h | 3.9 (3.6–4.0) 3.9 (3.3–4.0) | 4.2 (4.0–4.3) | < 0.001 |
| Urea [mg/dl] | Diagnosis 48 h | 26.7 ±11.98 24.4 ±12.69* | 28.1 ±6.92 | NS |
| Creatinine [mg/dl] | Diagnosis 48 h | 0.84 ±0.47 0.88 ±0.77 | 0.94 ±0.11 | NS |
| Calcium [mg/dl] | Diagnosis 48 h | 9.0 (8.7–9.2) 9.0 (9.0–9.2) | 10.1 (9.7–10.2) | < 0.001 |
| IL-35 [ng/ml] | Diagnosis 48 h | 5.91 (4.21–7.90) 6.79 (4.42–9.62)* | 25.53 (12.79–54.73) | < 0.001 |
| Pentraxin-3 [ng/ml] | Diagnosis 48 h | 6.75 (4.42–9.62) 6.75 (4.74–9.06) | 7.64 (6.58–8.62) | NS |
The relationship between aetiology and serum IL-35 and pentraxin-3 levels
As shown in Table IV, the median and interquartile range values of serum IL-35 levels in patients with AP due to biliary and idiopathic aetiology were 6.30 (4.66–8.11) ng/ml and 5.60 (3.63–6.81) ng/ml on admission and 6.87 (5.10–9.53) ng/ml and 5.98 (4.13–9.32) ng/ml at the 48th h, respectively. There was no significant difference between these groups. Alcohol and hypertriglyceridaemia aetiology groups were disregarded due to low sample size (2 patients in each group). The median and interquartile range values of serum pentraxin-3 levels in patients with AP due to biliary and idiopathic aetiology were 6.34 (4.26–8.92) ng/ml and 7.16 (4.54–10.97) ng/ml on admission and 6.85 (4.70–8.86) ng/ml and 6.41 (4.85–9.91) ng/ml at the 48th h, respectively. There was no significant difference between these groups.
Table IV
IL-35, PTX-3, and CRP levels according to AP aetiology and radiological findings
| Parameter | IL-35 | PTX-3 | CRP | |||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| Aetiology: | ||||||
| Biliary | 6.30 (4.66–8.11) | 6.87 (5.10–9.53) | 6.34 (4.26–8.92) | 6.85 (4.70–8.86) | 2.50 (0.81–5.72) | 2.87 (0.85–11.48) |
| Idiopathic | 5.60 (3.63–6.81) | 5.98 (4.13–9.32) | 7.16 (4.54–10.97) | 6.41 (4.85–9.91) | 1.60 (0.65–4.39) | 1.07 (0.49–5.58) |
| P-value¥ | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | 0.042 |
| Ultrasonographic findings: | ||||||
| Pancreatic oedema | 7.23 (4.87–17.57) | 8.80 (6.16–12.93) | 5.71 (4.22–8.86) | 5.42 (4.06–8.92) | 2.80 (1.01–10.22) | 2.35 (0.97–9.85) |
| Pancreatic oedema and peripancreatic fluid | 5.44 (4.72–6.54) | 6.66 (4.89–8.50) | 8.75 (4.92–9.25) | 6.95 (4.71–8.62) | 2.60 (1.34–4.94) | 6.85 (1.04–4.94) |
| Normal findings | 5.66 (3.40–6.87) | 5.64 (4.21–8.91) | 6.80 (4.42–10.63) | 6.75 (4.88–9.06) | 1.26 (0.58–3.76) | 1.23 (0.30–6.97) |
| P-value¥ | < 0.05 | < 0.05 | < 0.05 | < 0.05 | 0.026α | 0.014β |
*Mann Whitney-U test was used for analysis. Alcohol and hypertriglyceridaemia groups were disregarded due to low sample size (2 patients in each group).
Results of selected laboratory tests and correlations between serum IL-35 and serum pentraxin-3 levels
There were significant differences in serum amylase (592 U/l (292–1200) and 80 U/l (61–123), p < 0.001), lipase (1200 U/l (623–1994) and 67 U/l (36–173), p < 0.001), C-reactive protein (2.02 mg/ml (0.70–5.46) and 2.18 mg/ml (0.61–7.93), p = 0.008), leukocyte count (9.3 × 103 (7.1–11.4) and 7.98 × 103 (6.26–10.0), p = 0.001), neutrophil count (7.13 ±3.73 × 103 and 5.41 ±2.77 × 103, p < 0.001), lymphocyte count (1.78 ±0.88 × 103 and 2.13 ±1.39 × 103, p = 0.022), glucose (99.0 mmol/l (88.0–122.0) and 91.0 mmol/l (82.0–106.5), p = 0.006), and urea (26.7 ±11.98 mmol/l, 24.4 ±12.69 mmol/l, p = 0.001) levels on admission and at 48 h, respectively.
There were positive correlations between amylase and IL-35 levels on admission and at the 48th h (R = 0.358, p = 0.004 and R = 0.272, p = 0.018, respectively). There was positive correlation between lipase and serum pentraxin-3 levels at the 48th h (R = 0.523, p < 0.001). There was no correlation between serum IL-35 and pentraxin-3 levels at baseline and at 48 h and neutrophil and lymphocyte count (Table V).
Table V
Correlations between IL-35 and PTX-3 and selected biomarkers at 24 and 48 h from the onset of AP*
Discussion
The roles of the anti-inflammatory cytokine IL-35 and acute-phase protein pentraxin-3 have not yet been understood in AP patients. Our study demonstrates that, in patients with AP, serum IL-35 levels were lower than in the control group, and they represented a rising trend from baseline levels to 48-hour follow-up. We found no difference in terms of serum pentraxin-3 levels between patients and controls, nor between baseline and 48-hour levels.
It is known that atrophic acinar cells activate several inflammatory cells like macrophages and granulocytes, which release a number of pro-inflammatory cytokines such as IL-1, IL-6, IL-8, IL-18, IL-33, and TNF-α during pancreatic injury. There are several studies that shows higher levels of pro-inflammatory cytokines and the relation between systemic inflammatory response syndrome (SIRS) and multi-organ failure in AP [5, 15, 17]. Anti-inflammatory cytokines including IL-10, IL-1 receptor antagonist, and soluble IL-2 receptor are also reported to be significantly higher in patients with severe acute pancreatitis [18, 19].
IL-35 is a cytokine belonging to the IL-12 family, together with IL-12, IL-23, and IL-27. It consists of an IL-27p35 subunit and an Epstein-Barr virus-induced gene 3 subunit. IL-35 is an immunosuppressive cytokine mainly expressed by regulatory T cells (Treg), dendritic cells, macrophages, and monocytes [20]. There are several studies that show higher IL-35 levels in patients with sepsis, arthritis, multiple sclerosis, diabetes, and atopic dermatitis [9, 21–24]. Also there are several studies that represent lower levels of IL-35 in patients with rheumatoid arthritis, Hashimoto’s thyroiditis, and chronic immune thrombocytopaenia [13, 25, 26]. It is known that IL-35 exerts its immunosuppressive functions by promoting the differentiation of Treg and regulatory B (Breg) cells, inhibiting T-cell proliferation and T-helper 17 (Th17) differentiation, as well as manipulating the balance between Treg and Th17 [27, 28].
A study from China reported elevated serum IL-35 levels in patients with AP. In that study there were significant differences between severe and moderately severe AP and severe and mild AP patients. There was no significant difference between moderately severe and mild AP levels [29]. By contrast, in the present study serum IL-35 levels were lower in AP patients than in the control group. Although there was an increase in serum IL-35 level at 48 h, this value was also lower than in the control group.
Acute-phase proteins, especially CRP, are commonly used in the diagnosis of acute pancreatitis and in the evaluation of prognosis. The highest CRP levels were shown at 48–72 h in various studies. Serum CRP levels higher than 12–15 mg/dl were correlated with severe pancreatitis [30]. Both CRP and pentraxin-3 belong to the pentraxin family; CRP is the representative form of ‘short pentraxin’ while pentraxin-3 is ‘long pentraxin’ [16].
Although there are studies that indicate correlation between higher pentraxin-3 levels in patients with AP, our study did not confirm this finding. We found no correlation between control group and patients with AP or with serum pentraxin-3 levels at baseline and at 48 h. We found no difference in terms of serum CRP levels between baseline levels of AP patients and the control group, but there was a difference between baseline levels and serum CRP levels at 48 h (p = 0.008). This is probably due to all our patients having mild pancreatitis. In a recently published study, the levels of pentraxin-3 were significantly higher in patients with severe acute pancreatitis than in patients with mild and moderately severe AP (p = 0.014) [31]. Kusnierz-Cabala et al. also revealed higher serum pentraxin-3 levels in those with the severe compared to those with the mild form of AP (median 17.2 vs. 4.0 ng/ml on day 1, p = 0.03; 6.1 vs. 2.2 on day 5, p = 0.044) [32].
A major limitation of our study was the lack of a severe AP group, and the other is the medium sample size. To our knowledge, although this is the second study in which IL-35 was studied in patients with AP, it is the first one showing lower levels of IL-35 in patients with mild AP. At the 48-hour control an increase in baseline IL-35 levels was observed in mild AP patients. Again, although previous studies showed higher levels of pentraxin-3 in patients with AP, there was no difference in the pentraxin-3 level between the mild AP patients and the healthy control group in our study.
Conclusions
Interleukin-35 may be used in the diagnosis and follow-up in patients with mild AP, but pentraxin-3 should not. Future clinical studies including large numbers of AP and severe AP patients are needed to clear the diagnostic and prognostic value of IL-35 and pentraxin-3 levels in AP patients.