Introduction
Exocrine pancreatic insufficiency (EPI) is a clinical entity characterized by deficient exocrine pancreatic enzymes that results in altered digestion [1, 2]. Various causes of EPI have been identified. Causes of EPI can be broadly categorized into pancreatic or extrapancreatic diseases. Prior studies revealed that EPI can occur in the course of diabetes mellitus (DM), obesity and celiac disease [3]. Exocrine pancreatic insufficiency was also noted after upper gastrointestinal system surgery [4]. Beside pancreatic resections, gastric resections can induce EPI in differential pathological ways [5, 6]. Also type of gastric resection (subtotal versus total) and additional intestinal anastomosis techniques can influence the severity of EPI [7]. In EPI, especially lipase falls below 5–10% of normal level, steatorrhea, weight loss and symptoms associated with decreased quality of life levels occur. Suspicion and correct diagnosis are essential for EPI because enzyme replacement therapy frequently relieves the patient’s symptoms and quality of life.
Obesity and related complications are a major health problem in developed and also in developing countries. Beside diet, exercise and medical treatments, bariatric surgery gives a treatment opportunity for obese patients. Especially in morbidly obese patients, bariatric surgery achieves efficient weight loss and relieves obesity-related complications. Over the years, various surgical techniques have been reported within bariatric surgery with high clinical success. Nevertheless, beside their benefits they can lead to some morbidity including EPI. In the literature, several studies have investigated EPI after bariatric surgery. Among these, Borbély et al. investigated EPI after Roux-en-Y gastric bypass (RYGB) and they found a 48% EPI frequency in distal and 19% EPI frequency in proximal RYGB patients [8]. Unlike other bariatric surgery techniques, there is no knowledge about the sleeve gastrectomy (SG)–EPI association in the literature.
Material and methods
Study design and patient population
This is a prospective, case-control study which was conducted in the Gastroenterology and General Surgery departments of Kecioren Training and Research Hospital. Morbidly obese patients resistant to medical, diet and lifestyle therapy and therefore admitted for a sleeve gastrectomy procedure between January 2018 and April 2018 were enrolled in the study. Patients who had chronic pancreatitis, celiac disease, cystic fibrosis, diabetes mellitus, prior history of gastric and/or pancreatic surgery, prior history of acute/chronic pancreatitis and excessive alcohol consumption were excluded from the study.
Surgery procedures were done by an expert surgeon who had performed a total of nearly 1000 SG procedures. All SG procedures were done laparoscopically. Post-operative surgery related complications were not seen in any patients. Patients’ medical history, gastrointestinal complaints, laboratory parameters, transabdominal ultrasonography reports and also anthropometric measurement were recorded before and after the SG procedure.
Exocrine pancreatic insufficiency definition and fecal elastase-1 assessment
Exocrine pancreatic insufficiency was evaluated by the fecal elastase-1 test. Fecal elastase-1 levels > 200 μg/g were defined as normal, fecal elastase-1 levels < 200 μg/g were defined as EPI, with levels of 100–200 μg/g defined as mild EPI and < 100 μg/g defined as severe EPI. The first fecal samples were collected a week before surgery; the second fecal samples were collected in the third month after surgery.
Fecal elastase-1 concentrations of all samples were measured at the same time using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (BIOSERVE Diagnostics GmbH; Rostock, Germany) according to the manufacturer’s instructions. All fecal samples were collected under –20°C and then studied all together. Briefly, fecal samples were studied by an EL9 microplate reader and Model402 Microplate washer before fecal elastase-1 determination. Extraction buffer solution was added to fecal samples at a 10 mg/10 mg ratio. These samples were mixed in a vortex, and after a 20 s period supernatants of samples were collected for the study. Results were given as μg elastase/g stool.
Statistical analysis
The normality of distribution of continuous variables was tested by the Shapiro-Wilk test. The Mann-Whitney U test was used to compare 2 independent groups for non-normal data and the Wilcoxon test was used for non-normal data. The McNemar test was applied to investigate the relationship between categorical variable measured at 2 different time points. Statistical analysis was performed with SPSS for Windows version 24.0 and a p < 0.05 was accepted as statistically significant.
Results
A total of 40 patients were enrolled in the study. Of those, 37 patients were female and 3 patients were male. Mean age of patients was 41.9 ±11.25. The baseline laboratory parameters reveal normal hepatic and renal functions as shown in Table I. Overall 8 patients had gastrointestinal complaints (bloating in 5 patients, epigastric pain in 3 patients); other individuals were asymptomatic. According to their transabdominal ultrasonography, only 6 patients had pancreatic steatosis. Esophagogastroduodenoscopy was performed routinely before surgery and gastritis was the most common endoscopic finding; neither ulcer nor erosions were seen. Prior to surgery, the mean level of body mass index (BMI) was 47.29 ±6.14 kg/m2. Other mean anthropometric measurements were as follows: 125.35 ±14.17 kg for weight, 127.8 ±12.59 cm for waist circumference and 143.4 ±14.76 cm for hip circumference. After 3 months from surgery, all individuals were asymptomatic. Also obtained mean levels were 36.14 ±12.96 kg/m2 for BMI, 92 ±12.96 kg for weight, 94 ±12.97 cm for waist circumference and 119.45 ±14.6 cm for hip circumference (Table I). The post-operative third month levels of weight, BMI, waist and hip circumference were significantly decreased compared to pre-surgery levels (Table II).
Table I
Demographic characteristics, baseline laboratory parameters and anthropometric measurements of patients
Table II
Waist circumference, hip circumference, weight and BMI decreased significantly after surgery
| Variables | Preoperative (n = 40) | Postoperative (n = 40) | P-value |
|---|---|---|---|
| Waist circumference | 127.8 ±12.59 | 94 ±12.98 | 0.001* |
| Hip circumference | 143.4 ±14.77 | 119.45 ±14.6 | 0.001* |
| BMI | 47.3 ±6.14 | 36.14 ±5.26 | 0.001* |
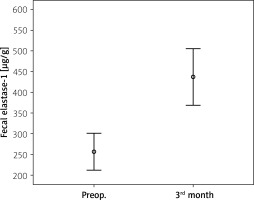
The mean pre-surgery fecal elastase-1 level was 256.25 ±137.16 μg/g and post-surgery level was 437.7 ±212.43 μg/g (p = 0.001) (Table III, Figure 1). Prior to surgery, 20 patients had reduced fecal elastase-1 levels between 100 and 200 μg/g. The other 20 patients had normal fecal elastase-1 levels. After surgery only 4 patients had reduced fecal elastase-1 levels between 100 and 200 μg/g, other patients had normal fecal elastase-1 levels. In these 4 patients, 3 patients already had reduced fecal elastase-1 levels, while only 1 patient had newly developed EPI (Table IV). Comparison of fecal elastase-1 between pre-surgery and post-surgery revealed a significant difference (p = 0.001).
Table III
Fecal elastase-1 was found significantly higher after surgery
| Variables | Preoperative (n = 40) | Postoperative (n = 40) | P-value |
|---|---|---|---|
| Fecal elastase-1 [μg/g] | 256.25 ±137.16 | 437.7 ±212.43 | 0.001* |
Table IV
Fecal elastase was found significantly higher after surgery
| Preoperative EPI frequency | Postoperative EPI frequency | P-value | |||
|---|---|---|---|---|---|
| Present | Absent | ||||
| Count | Row N % | Count | Row N % | ||
| Present | 3 | 15.0 | 17 | 85.0 | 0.001* |
| Absent | 1 | 5.0 | 19 | 95.0 | |
Discussion
Bariatric surgery is an effective treatment modality in obesity with new techniques and increased experience. Nowadays many patients are admitted to bariatric surgery clinics for morbid obesity and associated complications. Although weight loss is the primary aim of BS, surgery-associated morbidity and mortality are the leading limitations of BS procedures. Exocrine pancreatic insufficiency is one of them; prior studies demonstrate its increased frequency after RYGB [9]. However, there is no study investigating EPI after the SG procedure. Here, for the first time, we demonstrated that SG improves exocrine pancreatic functions.
Exocrine pancreatic insufficiency is a well-known complication of gastric surgery after gastric cancer. This type is a non-pancreatic cause of EPI. Various mechanisms can disrupt pancreatic secretions after gastric surgery including intestinal bacterial overgrowth due to decreased gastric acidity and altered denervation of the pancreas [10, 11]. Normal pancreatic exocrine functions need optimal pH in the intestinal lumen. Increased intestinal bacterial overgrowth due to decreased gastric acidity disrupts the normal pH value for optimal pancreatic secretions. Also decreased cholecystokinin (CCK) secretions lead to decreased exocrine pancreatic secretions in gastrectomy patients but also this mechanism is probably responsible for EPI after RYGB [12]. Sleeve gastrectomy preserves antrum-pylorus anatomical integrity. Thus, presence of antrum and gastrin maintains gastric acidity and optimal intestinal pH. This view can be the first explanation of our results. In addition, maintenance of anatomical integrity of the pylorus-duodenum is also an important process for normal pancreatic functions via normal CCK secretions and this is the second possible explanation of our results. In gastric bypass, digested foods are not interacted with gastric and pancreatic secretions and they pass small bowel without this interaction. Therefore exocrine pancreatic secretions via intestinal stimulation does not occur. In addition, CCK secretions diminish because of the ineffective stimulation of CCK-secreting cells. As a result, pancreatic secretions decrease and EPI occurs. In contrast with this situation, the pancreas maintains its active role in digestion after SG because of normal CCK stimulation.
Over the past two decades BS has become the most effective treatment modality for obesity. Compared to non-surgical treatment options such as medical treatments and exercise programs, weight loss and long-term protective effect from weight gain are more frequent in BS [13]. Several well-defined surgical techniques have been used in BS such as RYGB, SG, adjustable gastric banding, bilio-pancreatic diversion (BPD) and BPD with duodenal switch. Sleeve gastrectomy and RYGB are the two most commonly performed procedures. Roux-en Y gastric bypass is an effective and safe procedure [14]. Prior studies showed that the long-term success of RYGB in terms of weight loss and impaired metabolic complications is nearly 85%. Also type-2 DM and hypercholesterolemia were dramatically improved after the procedure. Nevertheless, there are several metabolic-intestinal and nutritional consequences after RYGB. Absorption of iron and vitamin D is affected after RYGB [15]. Exocrine pancreatic functions are also impaired after this procedure. With these metabolic-intestinal deteriorations, patients experienced several complaints associated with decreased quality of life. Sleeve gastrectomy is an effective procedure like RYGB in terms of weight loss. Compared to RYGB, nutritional deficiencies except vitamin B12 are extremely rare. This literature knowledge highlights that SG has better nutritional-intestinal consequences compared to RYGB. Also, based on our results, we can say that the SG procedure did not adversely affect exocrine pancreatic functions but also improves pancreatic functions.
It is well known that fecal elastase-1 is a simple, non-invasive test for the diagnosis of EPI [16]. In the medical literature, most studies used fecal elastase-1 to diagnose EPI. However, its accuracy is low despite its common use especially in severe EPI patients. There are several direct and indirect diagnostic methods for EPI but only the 13C-mixed triglyceride breath test is recommended by the FDA in clinical trials as a gold standard [17]. Recently, González-Sánchez et al. found that the diagnostic performance of fecal elastase-1 and 13C-mixed breath test was similar in chronic pancreatitis patients in terms of EPI diagnosis [18]. Beside this recommendation and in the light of this information fecal elastase-1 is still reliable for EPI diagnosis.
Our study has some limitations. We demonstrated early results after SG but we did not know long-term effects of the SG procedure on exocrine pancreatic functions. For this reason long-term follow-up studies are required. In our study group we did not assess pancreatic steatosis (PS) by advanced imaging techniques and this is another limitation. In obese patients it is possible to speculate that the frequency of PS may increase. Recently some studies have investigated the possible interplay between pancreatic steatosis and EPI. Kromrey et al. found that EPI was more common in patients with PS [19]. Although imaging modalities are not recommended as a diagnostic tool of EPI in current literature, investigating PS in our patients can better clarify the possible interaction between EPI and PS.
Conclusions
In our study, we found that SG relieves EPI in morbidly obese patients. Maintaining normal upper gastrointestinal system anatomical integrity is an important feature of SG and the possible explanation of our findings. Despite limitations of our study, this result contributes important knowledge to the literature.