Introduction
Bowel obstruction is an important cause of morbidity and mortality, accounting for nearly 30,000 deaths and more than $3 billion per year in direct medical costs; it is responsible for approximately 15% of hospital admissions for acute abdominal pain in the USA and ~20% of cases needing acute surgical care [1]. Bowel obstruction aetiology is based on a mechanical intrinsic luminal obstruction or extrinsic compression. Adynamic ileus and colonic pseudo-obstruction are caused by a lack of enteric propulsion [2]. Colonic pseudo-obstruction and adynamic ileus can be caused by drugs, trauma, postoperative period, metabolic disturbance, and other different bases [2, 3]. The concept management of patients with small bowel obstruction (SBO) became more complicated in 1981 when Bizer et al. reported that nonoperative management was successful in a significant percentage of patients [4]. The approach has changed considerably since then because of advancements in imaging technology, the prevalence of adhesion disease, the prominence of laparoscopy, and the development of protocols to help ensure timely intervention. What has not changed is the need to avoid nontherapeutic surgery, as well as any unnecessary delay when surgery is required. Morbidity and mortality due to SBO increase when there is an undue delay in operation and decrease with the institution of appropriately timed surgery [5]. SBO is caused mainly by postoperative adhesions (more than 75% of all cases) [6–10]. The operative procedures usually associated with SBO are colectomy, hysterectomy, and appendectomy [11]. Other causes of SBO are Crohn’s disease (7%), neoplasm (5–10%), hernia (2%), or radiation-induced enteritis (1%) [6–9]. In a series of 29,790 patients with a single previous abdominal or pelvic surgery, Ellis et al. reported that within the following 10 years 34.6% of them were readmitted a mean of 2.1 times for a disease related to adhesions [12]. About 10% of patients have ‘spontaneous’ SBO with no previous abdominal surgery [11].
Adhesive small bowel obstruction (ASBO) is one of the leading causes of surgical emergencies and in particular of surgical emergencies that require an emergent operation [13–15]. ASBO causes considerable harm, resulting in 8 days of hospitalization on average and an in-hospital mortality rate of 3% per episode [16–20]. Between 20% and 30% of patients with adhesive small bowel obstruction require operative treatment [13, 21, 22]. The length of hospitalization and morbidity depend on the need for surgical intervention. The average hospitalization time after surgical treatment of ASBO is 16 days, compared to 5 days following non-operative treatment. Associated costs in a Dutch study in 2016 were estimated at €16,305 for surgical and €2227 for non-operative treatment [23]. Therefore, the World Society of Emergency Surgery (WSES) working group on ASBO has developed evidence-based guidelines to support clinical decision-making in the diagnosis and management of ASBO [22, 24].
Patients with incurable, advanced abdominal or pelvic malignancy often present to acute surgical departments with symptoms and signs of intestinal obstruction. It is rare for bowel strangulation to occur in these presentations, and spontaneous resolution often occurs, so the luxury of time should be afforded while decisions are made regarding surgery. Cross-sectional imaging is valuable in determining the underlying mechanism and pathology. The majority of these patients will not be suitable for an operation and will be best managed in conjunction with a palliative medicine team. Surgeons require a good working knowledge of the mechanisms of action of anti-emetics, anti-secretories, and analgesics to tailor early management to individual patients, while decisions regarding potential surgery are made. Deciding if and when to perform operative intervention in this group is complex and fraught with both technical and emotional challenges. Surgery in this group is highly morbid, with no current evidence available concerning quality of life following surgery [25]. The Eastern Cooperative Oncology Group/World Health Organization Performance Status (ECOG/WHOPS) is a prognostic factor. It was used in analysing health outcomes such as risk-adjusted hospital performance models in cancer populations. Performance status is rarely recorded in surgery, often the place where cancer is first diagnosed. This review article pondered the role of the prognostic score index in the management of small bowel obstruction.
Anatomy and pathophysiology of small bowel obstruction
When considering SBO, it is important to understand the difference between functional disorders that lead to non-propulsion through the gut and mechanical disorders that impede otherwise normal propulsive effort. Gastrointestinal paralysis (ileus) secondary to enteritis that may be attributable to surgery, medication, infection, or inflammation is the most common imitator of SBO in terms of presenting symptoms, physical examination findings, and static imaging findings. It is often brought to the attention of a surgeon when a radiologist states that SBO cannot be ruled out based on radiographic patterns. Ileus results in dysfunctional peristalsis, which is not correctable with surgery, and it often falls on the surgeon to differentiate between the two. Relevant history including identification of risk factors for ileus, trends in the abdominal examination and laboratory results, and dynamic contrast imaging findings help to make the call [26].
Diagnosis of small bowel obstruction
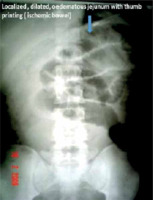
Clinical presentation of pain, vomiting, distension and constipation, and laboratory and radiographic factors should all be considered when making a decision about treatment of bowel obstruction [27]. One must rule out an abdominal wall hernia as a cause of bowel obstruction, which is seen in 26.8% of cases in virgin abdomen [28]. Plain radiograph should be an integral part of management of patients with clinical suspicion of bowel obstruction and gastrointestinal perforation (Figure 1) [29]. The diagnosis in most cases will be confirmed by further imaging studies such as ultrasound, contrast studies, or, most commonly in contemporary practice, computed tomography (CT) [30]. The CT scan, besides confirming the diagnosis of bowel obstruction, gives information on partial or complete obstruction and its location, it also provides the specific type, e.g. closed loop type, and helps in deciding early surgery. Contrast enhanced computed tomography (CECT) gives enough information on ischaemic bowel and bowel oedema, which requires emergency surgery, and luminal Gastrografin helps in relieving the bowel obstruction [31, 32]. Surgeons find coronal images more helpful than axial images for management [33]. The radiographic transition zone alone does not increase the likelihood of surgical intervention or identify patients who will fail non-operative management [34]. The 4 cardinal features: intra peritoneal free fluid, mesenteric oedema, presence of the “small bowel faeces sign”, and history of vomiting, are predictive of requiring immediate emergency operative intervention [27].
Decision for surgery
Timing is crucial to avoid gangrenous bowel resection and obvious electrolyte imbalance. Identifying patients who may safely undergo non-operative management remains difficult. A study by Leung and Vu revealed that out of 1613 patients 56.6% required surgery and 43.4% could be managed non-operatively. There was an associated higher incidence of bowel resection in patients who took increased time to reach the operating room. Among the patients in whom the admission to operating room was less than 24 h, 12% of patients had bowel resection, as compared to 29% of patients who took more than 24 h [35]. To avoid potentially increasing the risk of bowel loss, intervention should be considered by the second day in paediatric patients with low threshold, in those who do not exhibit signs of improvement, and no more than 5 days in adults [36, 37]. Patients on conservative treatment for BO, in whom the drainage volume through the nasogastric tube on day 3 is > 500 ml, mostly required surgery [38]. CT scan of the abdomen with oral Gastrografin not only gives the location of BO but also adds to the Gastrografin trial and avoids abdominal surgery [39].
SBO prediction score index models
Zielinski et al. published a model based on a detailed retrospective review of patient records that used data from the available clinical scenario of the patient at the time of admission. The model developed was successful in predicting the need for operative intervention in the setting of SBOs. The goals of that study and the model generated were to prevent unrecognized strangulation obstructions and to identify patients who would require operative treatment, in an effort to improve patient outcomes and decrease the duration of hospital stays. The model was based on the presence of 4 clinical features that were predictive, in multivariate analysis, of the need for operative intervention during that hospitalization. These 4 features were (i) history of vomiting and features on CT of (ii) intraperitoneal free fluid, (iii) mesenteric oedema, and (iv) lack of the small-bowel faeces sign. When all 4 signs were present, use of the model would have demonstrated a dramatic improvement in mortality with early operative exploration [27].
Zielinski et al. conducted prospective, observational validation of a multivariate SBO model to predict the need for operative intervention. Data from 100 consecutive patients with small-bowel obstruction and concurrent CT were collected prospectively by the authors, and data were analysed using appropriate statistical methods. This new prospective model composed of 3 features viz. history of obstipation, mesenteric oedema, and lack of small-bowel faecalisation maintained the same degree of discrimination (c-index of 0.77 vs. 0.75 for the original model) while simplifying the model to these 3 features. The greatest benefit that this model appears to offer over many other algorithms is the ability at admission to predict the need for eventual operative intervention during the hospitalization. Additionally, the ability to identify those patients who may not have strangulation obstruction but who will require operative intervention before dismissal for failure of the SBO to resolve can prevent delays of operative management and should decrease total hospitalization by eliminating the preoperative days of nonoperative, expectant management [40]. According to Jeffrey et al., the early post-operative mortality is closely linked with the age and the American Society of Anesthesiologists (ASA) grade, and the long-term mortality with post-operative complications [41]. Duron et al. revealed that more frequent bowel resections might be suggested for patients featuring 10 or more obstructive strictures and an intestinal wall injury, especially when associated with a reversible intestinal ischaemia [42]. In a narrative review of the literature pertaining to common age-related aetiologies, diagnosis methods leading to standard decision-making and treatment of acute intestinal obstruction reported by Pujahari revealed that predicting the conservative or operative management in SBO is difficult. The decision about surgery should be made in paediatric patients within 24 h, in young age, in virgin abdomen, and large SBO by 48 h, and within 3–5 days of admission in adults, if the oral Gastrografin fails to resolve the SBO more so the adhesive obstruction with high (> 500 ml) gastric tube aspirate (Algorithm). In recurrent SBO some form of plication may be considered during surgery. The early post-operative mortality is closely linked with the age and the ASA grade whereas the long-term mortality is associated with post-operative complications [43].
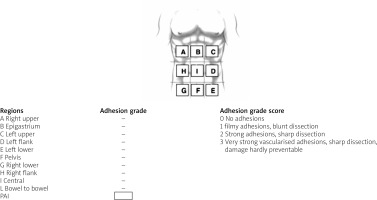
The most frequently used classification of adhesions in general surgery is the adhesion score according to Zühlke et al. (Table I) [44]. The score is based on the tenacity and some morphologic aspects of the adhesions. The merits of this score are that it is easy to use and the classifications are self-explanatory to most surgeons and gynaecologists. The major drawback to the score is that it does not measure the extent of adhesions and that the tenacity of adhesions can vary between different parts of the abdomen [45]. A recently introduced score by the ASBO working group is the peritoneal adhesion index (PAI), which measures tenacity on a 1–3 scale at 10 predefined sites, to integrate tenacity and the extent of adhesions in a single score (Figure 2) [46]. This score is the only score that has been validated to be prognostic for convalescence after surgery for ASBO and the risk of injuries during adhesiolysis [47]. A limitation to all these adhesion scores is that they are only applicable to operative cases because they require operative assessment. Furthermore, none of them has yet been validated to correlate with the long-term risk for (recurrence of) adhesion-related complications. A different type of classification in the field of ASBO is risk stratification that predicts the need for surgery. Zielinski reported on 3 radiological and clinical signs that correlate with the need for surgical exploration: mesenteric oedema, absence of the small-bowel faeces signs, and obstipation. The score was validated in 100 cases of ASBO and predicted the risk with a concordance index of 0.77 [40]. A more accurate model was reported by Baghdadi et al., whose score comprises radiological findings, sepsis criteria, and comorbidity index. Although the score is somewhat complex to assess, it correlates with an area under the curve of 0.80 in a validation study of 351 cases [48]. Henry et al. developed A nomogram scoring system was developed from factors that correlated with increased 30-day mortality to create a tool to determine short-term mortality for those patients presenting with malignant bowel obstruction (MBO) independent of therapy. Five factors found to be predictors of 30-day mortality were assigned a value of 1 if present or 0 if not. A score of 0–5 was then assigned to each patient based on the sum of these factors. Using the 5 risk factors of ascites, carcinomatosis, complete small bowel obstruction on imaging, hypoalbuminaemia, and abnormal white blood cells count (WBC), we evaluated 30-day mortality for the 498 patients with MBO, who had a known survival status at 30 days. While most of the patients had 0–2 risk factors, more than 30% of patients presenting with MBOs had ≥ 3 risk factors. Patients who had no risk factors present at the time of admission had the lowest mortality rate. Mortality increased significantly as the number of risk factors increased (p < 0.001). Henry et al. established a second scoring system to help determine which patients with an acceptable short-term survival would benefit from surgery. To do so, the authors compared between those treated surgically and non-surgically. As stated above, there were considerable differences between these 2 groups that would make it difficult to determine prognostic factors. Therefore, propensity scoring was implemented with 8 factors: age, gender, carcinomatosis, complete small bowel obstruction, ascites on imaging, leukocytosis, hypoalbuminaemia, and cancer type. This resulted in a subgroup of 226 patients (113 per group) who were more similar. Thus, Henry et al. two nomogram scoring system that would guide decisions in the care of patients with malignant bowel obstruction, and these nomograms are able to predict 30-day mortality and ascertain who may benefit from surgery for small bowel obstruction [49]. Huang et al. aimed to develop a model for predicting the risk of strangulated small-bowel obstruction (SSBO) because early and accurate diagnosis of SSBO is difficult. The authors used a database of 417 patients who had clinical symptoms of intestinal obstruction confirmed by CT, who were evaluated for inclusion in this study. The symptoms and laboratory and radiological findings of these patients were collected after admission. These clinical factors were analysed using logistic regression. A logistic regression model was applied to identify determinant variables and construct a clinical score that would predict SSBO. The findings of this study revealed that 26 patients were confirmed to have SSBO, 169 patients required surgery but had no evidence of intestinal ischaemia, and 172 patients were successfully managed conservatively. In multivariate logistic regression analysis, body temperature ≥ 38.0°C, positive peritoneal irritation sign, white blood cell (WBC) count > 10.0 × 109/l, thick-walled small bowel ≥ 3 mm, and ascites were significantly associated with SSBO. Thus, a new prediction model with total scores ranging from 0 to 481 was developed with these 5 variables. The area under the curve (AUC) of the new prediction model was 0.935. The prediction model devised by Huang et al. was a good predictive model to evaluate the severity of SBO [50].
Table I
Classification of adhesions