Introduction
Diabetes mellitus (DM) is a heterogeneous disease, of which type 1 DM (T1DM) is characterized by absolute lack of insulin, which mainly results from autoimmune destruction of pancreatic beta cell mass. In several cases, in spite of a strong inheritance (type 1B), the cause of beta cell destruction is unknown [1].
The gastrointestinal tract harbours a complex and dynamic population of microorganisms, known as the gut microbiota, which exert a marked influence on the host’s homeostasis and diseases. Vaarala et al. suggested that the interaction between the intestinal environment, the barrier function, and the immune system are crucial in the onset of T1DM. The gut microbiota modulates the function of the gut immune system by its effect on the innate immune system, such as the intestinal epithelial cells and dendritic cells, and on the adaptive immune system, in particular intestinal T cells. Due to the immunological link between gut and pancreas, e.g. the shared lymphocyte homing receptors, the immunological changes in the gut are reflected in the pancreas [1, 2].
In early experimental autoimmune diabetes before the development of insulitis, altered gut microbiota and altered immunostasis were paralleled by abnormalities of the gut barrier, leading to increased intestinal permeability and the transit of antigens. Bacterial antigen passage into the circulation evokes an immune reaction, affecting beta cells of the pancreas and causing insulin deficiency [3–5].
A study by Bosi et al. suggested that increased gut permeability preceded the clinical onset of T1DM [6]. Reverting dysbiosis to the normal gut composition can theoretically reduce the risk of T1DM development. Several animal studies have shown that probiotic administration can have promising effects on the control of T1DM [7]. An example of this was reported in a study by Dolpady et al. (2016). These authors administered a mixture of several Bifidobacteria and Lactobacilli at the time of weaning and afterwards to NOD mice and found that these probiotics prevented insulitis and autoimmunity through the reduction in the number of T-helper 1 (Th1) and T-helper 17 (Th17) cells in the intestinal mucosa and pancreatic lymph nodes. Based on these studies, it has been suggested that the administration of probiotics could be a measure of T1DM primary prevention [8].
Lactobacillus reuteri (L. reuteri) is a well-studied probiotic bacterium that can colonize different body sites, including the gastrointestinal tract. Notably, the decrease in the abundance of L. reuteri in humans in the past decades is correlated with an increase in the incidences of inflammatory diseases over the same period. Some L. reuteri strains can reduce the production of pro-inflammatory cytokines while promoting regulatory T cell development and function. In addition, the colonization of L. reuteri may decrease the microbial translocation from the gut lumen to the tissues. Microbial translocation across the intestinal epithelium has been hypothesized as an initiator of inflammation. Therefore, inflammatory diseases may be ameliorated by direct supplementation or prebiotic modulation of L. reuteri [9, 10].
Aim
The present study was designed to identify and quantitate some gut bacteria, i.e. Bacteroides, Prevotella, Ruminococcus, Lactobacilli, Lactobacillus johnsonii, Lactobacillus reuteri, and Veillonella, which were previously hypothesized to be associated with T1DM. This may have an impact on our future understanding of the pathogenesis of T1DM and possible approaches to prevent and treat it.
Material and methods
Patients
The present study was carried out in Alexandria Main University Hospital. The study included 40 T1DM patients who were recruited from the Diabetes Outpatient Clinic, and 20 healthy subjects with matched age, sex, body mass index (BMI), and dietary habits as a control group.
Exclusion criteria
Patients were excluded if they had any other acute or chronic inflammatory diseases or infectious diseases at study entry. The study participants received no antibiotic treatment, probiotics, prebiotics, or any other medical treatment influencing intestinal microbiota during the 3 months before the start of the study. Also, patients with chronic liver or renal diseases, in addition to those with other autoimmune diseases, were excluded from the study.
Ethical approval
The study follows the principles of the Declaration of Helsinki. After approval of the Ethical Committee (approval number: 0105301), Faculty of Medicine, Alexandria University, signed informed consent was obtained from each patient, expressing their acceptance to participate in the study and have the results published.
History
Detailed history was taken from patients and controls, with special emphasis on dietary history, smoking, and drug history.
Clinical examination
All patients and controls were subjected to a full clinical examination. Body weight and height were measured, and BMI was calculated.
Laboratory investigations
Laboratory investigations included fasting blood sugar (FBS) level and glycosylated haemoglobin (HbA1c).
Microbiome study
Specimen collection, preservation, and transport
Stool specimens were collected from cases and controls, kept in a freezer upon defecation at home, delivered to Alexandria University Main Microbiology laboratory frozen, and stored at –80°C until DNA extraction in the same week.
DNA extraction
DNA was extracted from 180-mg stool samples using a QIAamp DNA Stool Extraction Mini Kit (Qiagen, Germany).
SYBR Green Real Time PCR
Specific Oligonucleotide primers were used to target the 16S rRNA gene (rDNA) sequences of Bacteroides, Prevotella, Ruminococcus, Lactobacillus johnsonii, Lactobacillus reuteri, and Veillonella. Primers were also used to amplify a conserved 16S rDNA sequence present in all bacteria (universal primer set, recognizing domain bacteria), the amplification of which served as the denominator against which the amplification of the other bacteria was compared. All the primer sequences were derived from previously published studies [11–17]. Primers were commercially obtained (Metabion International AG, Germany).
Amplification was performed in a light cycler (Rotor Gene Q, Qiagen, Germany) using a SensiFASTTM SYBR No-ROX PCR kit (Bioline Co., UK). In short, forward and reverse primers (4 pmol each) were used in 20-µl reactions containing 2 µl of the DNA extract.
PCR amplification was performed with initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. Melting curve analysis was performed from 40 to 95°C with a plate-reading step after every 1°C and held at a temperature for 10 s to check the specificity of the product formed. Quantitation of specific bacterial DNA was expressed as relative quantitation (the cycle threshold (Ct) at which DNA for a specific target was detected relative to the cycle threshold (Ct) at which universal bacterial DNA was detected). This relative quantification is calculated automatically by the Rotor Gene software and expressed as relative fold difference [17].
Statistical analysis
Data entry and analysis were carried out using the Statistical Package for Social Sciences version 20 (SPPS PASW Statistics, Chicago). Data were coded, entered, and code checked before analysis. Quantitative variables were presented in the form of range, mean, median, and standard deviation. On the other hand, the studied qualitative variables were presented as frequency and percentage from the total. Comparisons between the different study groups were carried out using the χ2 test for qualitative variables and t-test for quantitative variables. All results were interpreted at a 5% level of significance where the difference between the study groups is considered significant if p ≤ 0.05.
Results
Clinical and demographic data of T1DM patients
Forty T1DM patients were enrolled in the study, with mean age ± SD 25.9 ± 5.9 years, male to female ratio 1 : 1, mean weight 67.3 ±8.039 kg, height 1.69 m, and mean BMI 23.39 kg/m2. The mean disease duration was 17.62 ±6.43 years. The mean HbA1c was 7.72 ±0.549, and mean fasting blood sugar (FBS) was 258.07 ±87.99 mg/dl.
Out of the 20 control cases examined there were 10 (45.5%) males and 12 (54.5%) females, with a female to male ratio of 1.2 : 1. The mean age ± SD of the cases was 32.3 ±5.57.
SYBR Green Real Time PCR assay results
Quantitation of specific bacteria DNA is not expressed as an absolute number but rather relative to total bacteria DNA present in the stool sample. Mean relative difference values of the various bacteria are shown in instances when the decimal value is low; exponential values are shown as E-05 (e.g. 4.74 × 10–5 is shown as 4.74E-05)
By quantitation of Bacteroides, Prevotella, Ruminococcus, Lactobacillus johnsonii, Lactobacillus reuteri, and Veillonella, as shown in Table I and Figures 1 and 2, T1DM patients showed significantly higher Bacteroides (p < 0.001) and Lactobacillus johnsonii (p = 0.003) but lower Veillonella than the control group (p = 0.013).
Table I
Comparison of the bacterial relative abundances in the study groups
Figure 1
Comparison between the studied groups regarding gut microbiome profile
*Statistically significant at p ≤ 0.05.
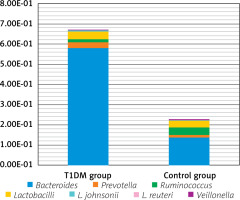
Figure 2
Box and whisker graph of the gut microbiome in the studied groups; the thick line in the middle of the box represents the median, the box represents the inter-quartile range (from 25th to 75th percentiles), the whiskers represents the minimum and maximum

However, there is no statistical difference between T1DM and control cases as regards Prevotella (p = 0.204), Ruminococcus (p = 0.598), Lactobacilli (p = 0.901), and Lactobacillus reuteri (p = 0.332). Regarding Lactobacillus reuteri, it was found only in 3 T1DM (7.5%) patients and in 4 (20%) control cases.
Table II shows a comparison between L. johnsonii-positive and -negative cases in the T1DM group with different variables and other studied bacteria. The L. johnsonii-positive group has statistically significant lower Prevotella (p = 0.04), Ruminococcus (p = 0.001), and Veillonella (p = 0.045) than the L. johnsonii-negative group.
Table II
Comparison between L. johnsonii-positive and -negative T1DM cases
There was no statistically significant correlation between the different bacteria of the T1DM cases and different variables, which are as follows: age, gender, disease duration, HbA1c, and FBS, except for Bacteroides, which showed a statistically significant negative correlation with BMI (R = –0.256, p = 0.049). Also, the Prevotella/Bacteroides ratio (P/B) showed a statistically significant positive correlation with BMI (R = 0.287, p = 0.027).
There was no statistically significant difference between the different gut bacteria of the control cases and the different variables, which are as follows: age and gender, except for Ruminococcus, which was significantly higher in females (p = 0.033).
Discussion
The overall results of the present study agree with previous studies reporting that patients with T1DM exhibit microbial dysbiosis. Bacteroides and Lactobacillus johnsonii were significantly increased in patients with T1DM compared to controls, while the relative abundance of the beneficial bacteria associated with the gut barrier and anti-inflammatory state, Veillonella, was significantly decreased. These bacterial differences could be responsible for the altered gut permeability previously described in patients with T1DM.
Regarding Bacteroides, the present study agrees with many studies, such as Leiva-Gea et al. (2018), who showed significantly higher relative abundance of Bacteroides in T1DM patients compared with healthy controls [18]. Similar results were reported by Murri et al. (2013), Lopez-Dominguez et al. (2016), and Huang et al. (2018), and this is of relevance because Bacteroides have been associated with gastrointestinal inflammation and increased intestinal permeability [16, 19, 20].
Regarding Prevotella, the present study demonstrated that the gut of T1DM patients harbours a higher level than the normal controls, but the difference was statistically insignificant. This result agrees with a recent study done in Egypt reporting that Prevotella was significantly higher in their T1DM cases [21]. In contrast to the present study, Brown et al. (2011) reported that the Prevotella level was lower in T1DM patients than in healthy unrelated controls [22]. Mejıa-Leon et al. (2014) showed that Prevotella is greatly decreased in autoimmune diseases associated with gut dysbiosis; however, the results were statistically insignificant [23]. Wu et al. (2011) attributed the increase in Prevotella to consumption of a diet rich in carbohydrates and plant fibres [24]. Bacteroides-dominant gut communities were also observed in prediabetic Finish children, who also showed decreased levels of Prevotella when compared to healthy controls [22].
As regards Ruminococcus, the present study demonstrated that T1DM patients had statistically insignificant lower levels than the normal controls. Similarly, Huang et al. (2018) showed that Ruminococcus is more abundant in healthy controls than in T1DM patients [20].
Regarding Lactobacilli, the present study demonstrated that T1DM patients had statistically insignificant higher levels than the healthy controls. Alkanani et al. (2015) reported that Lactobacilli levels were higher in T1DM patients than in healthy controls [25]. A high level of Lactobacilli has been associated with beneficial effects on proinflammatory disorders [26]. Support for the possibility that Lactobacilli potentially down modulates inflammation is provided by data that dendritic cells cultured with species of Lactobacilli induce polarization of regulatory T cells [27, 28].
Several modes of action have been proposed for probiotics such as Lactobacilli, including strengthening of the intestinal epithelial barrier function by stimulation of mucin secretion or enhancement of tight junction function, the clearance of pathogens by competitive binding to receptors presented by epithelial cells, production of anti-inflammatory compounds, and the synthesis of antimicrobial substances such as bacteriocins. Another key mode of action by which probiotics are proposed to exert their beneficial effects is through modulation of the host immune system in the intestinal mucosa [27].
Individual species of the gut bacteria may have different effects on T1DM. Using the Bio-Breeding (BB) rat model, Lactobacillus johnsonii, which was isolated from Bio-Breeding diabetes-resistant (BB-DR) rats, prevented diabetes development in Bio-Breeding diabetes-prone (BB-DP) rats whereas Lactobacillus reuteri failed to affect diabetes development [14]. Lactobacillus johnsonii affects epithelial integrity directly, and causes induction of IL-17 immunity in the mesenteric lymph nodes and spleen [4, 29].
As regards, Lactobacillus johnsonii, the present study demonstrated that T1DM patients had significantly higher levels than the healthy controls. For Lactobacillus reuteri, there was no statistically significant difference between T1DM and control cases (p = 0.332). It was found only in 3 T1DM (7.5%) patients and in 4 individuals from the control group (20%). Although L. reuteri occurs naturally in humans, it was not found in all our participants [30].
In contrast to the results of the present study, other studies reported that Lactobacillus johnsonii has a protective effect in relation to T1DM. The results of Roesch et al. (2009) were consistent with the concept that beneficial bacteria seem to provide a protective effect in rodent models by delaying or preventing the onset of diabetes. Because BB-DP rats have lower populations of species that contain known probiotic strains than do BB-DR rats, potentially beneficial bacteria may be necessary for the maintenance of a healthy microbiome, which is essential in preventing a leaky gut [31]. Valladares et al. (2010) and Lau et al. (2011) reported that BB-DP rats, when orally fed with Lactobacillus johnsonii, became resistant to the onset of T1DM, whereas the Lactobacillus reuteri strain did not [14, 32].
However, the case is different in the present study as regards our patients’ age and stage of the disease, and these researchers were dealing with an animal model.
As regards Veillonella, the present study demonstrated that T1DM patients had significantly lower levels than the healthy controls. In contrast to the present study, Brown et al. (2011) stated that Veillonella can compete for lactate substrate with the butyrate producers and are in statistically higher abundance in cases than in controls [22]. Also, Murri et al. (2013) and Radwan et al. (2020) in Egypt reported significantly high levels of Veillonella in T1DM patients [16, 21].
Many studies have demonstrated that the altered abundance of specific members or reduced diversity of gut microbiota was associated with the progression of T1DM. However, the exact role of the gut microbiota in the pathogenesis of T1DM remains controversial. Up to now, the most convincing evidence for a causal link between intestinal microbiome and the disease comes from well-controlled intervention studies in murine models. These studies illustrated the efficacy of probiotic supplementation, antibiotic use, faecal microbiota transplantation (FMT), and diet intervention in modifying the risk of T1DM via changing the gut colonization patterns [33].
The limitations of this study are mainly reflected in the following 2 points. Firstly, the sample size is relatively small. The results should be confirmed in a larger sample and among patients of different courses (initial and long course) of type 1 diabetes mellitus in future to determine dysbiosis at time of autoimmune insult. Secondly, the number of bacteria detected, comprising many bacterial species, may be more accurate to determine if there are possible associations between the gut microbiome and T1DM.
Conclusions
Egyptian patients with T1DM showed dysbiosis of the gut microbiome, which approximately related to that of the autoimmune diseases pattern. This highlights an important relationship between gut microbial dysbiosis and T1DM. Further large studies may determine if there are any other possible associations between the gut microbiome and T1DM.










