Introduction
Hepatocellular carcinoma (HCC) is among the five leading causes of cancer-related death worldwide, with mortality rates reaching 6% and with a reported 5-year survival of 18% [1, 2]. Despite the fact that previous SEER registries reported expected HCC rates to continuously increase in the forthcoming decades, the latest data demonstrates that HCC rates declined significantly between 2011 and 2016 [2]. However, taking in consideration that risk factors for HCC are widely known and strict screening protocols are usually implemented to identify early-stage HCC, 70% to 80% of patients are still diagnosed at an advanced stage [3]. Hepatocellular carcinoma is characterized by its propensity to invade the vasculature within the liver, especially portal vein tributaries or even the main trunk of portal vein. Portal vein tumour thrombosis (PVTT) is the most common form of macrovascular invasion of HCC with a prevalence rate ranging from 10% to over 60% [4–8] and median survival of 2.7–4.0 months, when the tumour remains untreated [8, 9]. On the other hand, a fact that needs to be emphasized is that many cases of portal vein thrombosis in HCC patients are not the result of tumour thrombus. More specifically, cirrhotic patients usually present with non-neoplastic portal vein thrombosis with an incidence that ranges between 0.6% and 11%, and therefore prompt differentiation from PVTT is of great importance [5].
The prognosis of patients with HCC is multifactorial and depends on both tumour and liver factors [10]. Tumour diameter, multifocality, PVTT, and α-fetoprotein (AFP) blood levels are the most important factors related to prognosis. Among these, PVTT reflects tumour aggressiveness and limits standard treatment options such as liver resection or transplantation. In addition, consequences on residual liver function cannot be overlooked [7, 11]. According to the treatment guidelines of the American Association for the Study of Liver Disease/Barcelona Clinic for Liver Cancer (AASLD/BCLC) Staging System, PVTT is considered an advanced stage of the disease with dismal prognosis, and the proposed treatment options are limited nowadays to chemotherapy, with a median survival time of 10.7 months [6, 12]. Multiple treatment options exist in treating small HCCs in well compensated cirrhosis, classified as extremely early and early stage HCCs. Among these, surgery is seemingly feasible and effective, with more favourable outcomes when compared to non-surgical approaches, achieving 77.2% to 91.5% overall survival [13, 14]. However, to date, the surgical strategy for HCC with PVTT remains controversial. As a result of recent advances in surgical techniques and perioperative management, aggressive surgical resection for HCC with vascular invasion has been proposed, to improve the survival benefit in this group of patients [12, 15–18]. In real-life experience, the adherence to international guidelines for HCC treatment is far from being universally applied, with many surgeons worldwide following personalized approaches on a case-by-case basis. It seems that there is scarce evidence of survival benefit provided by a therapeutic approach of HCC beyond the guidelines if an individualized approach is implemented. Because the nature of advanced HCC remains heterogeneous, there is a need to expand the treatment options of individualized therapeutic strategies applied in selected group of patients [12, 15].
Material and methods
A thorough literature search in PubMed and Google Scholar, under the terms ‘hepatocellular carcinoma AND portal vein thrombosis’ until 31 December 2020, regarding the surgical management of portal vein thrombosis was conducted by the authors, and the associated results are presented in this narrative review.
Discussion
Current stage classification of HCC and controversies of clinical guidelines
Different staging systems exist regarding disease stage classification associated with prognosis and survival in patients with HCC. Each stage has been linked with specific treatment guidelines, and different treatments are offered to different subgroups of patients [19, 20]. Liver transplantation (LT), surgical resection, and ablation techniques are considered the most effective treatment modalities, which benefit patients with an early-stage tumour (BCLC 0/A). Patients with greater tumour burden confined to the liver (stage BCLC B-C), who are not indicated for radical treatments, could still benefit from local treatments as arterial chemo-radioembolization (TACE-TARE) or oral treatment with the multi-kinase inhibitor (sorafenib) [20]. However, therapeutic algorithms for HCC recommended by international study groups depend on several parameters, such as the scarcity of liver donors for LT, identification or not of early tumours, and the performance status of the patients. These factors play a pivotal role in the risk benefit ratio when non-transplant curative treatments are implemented [20]. Most staging systems classify HCC with PVTT as an advanced-stage disease. Non-surgical treatments, including molecularly targeted therapy, TACE, TARE, or best supportive care, are the main therapeutic methods used in many centres, especially in the West [6]. Moreover, the European Association for the Study of the Liver (EASL) and the American Association for the Study of the Liver (AASLD) guidelines classify HCC with PVTT based on Barcelona Clinical Liver Cancer criteria (BCLC) as stage C, and recommend systemic therapy with sorafenib [14, 21–23]. According to this algorithm, liver resection is not the optimal treatment option for patients with advanced-stage disease. Instead, surgical management is limited to patients with early-stage tumours [24].
On the other hand, there are scientific studies showing that liver resection for HCC with PVTT could provide significant survival benefit and may be advantageous in terms of avoiding liver failure secondary to tumour thrombus [12, 25–27]. Under this view, most medical centres in Asian countries, which have the highest HCC prevalence worldwide, follow approaches expanding EASL/AASLD guidelines. East-Asian countries, through a multidisciplinary approach, have expanded the indications for surgery with satisfactory outcomes in selected patients with BCLC stage C against sorafenib monotherapy [22, 28, 29]. According to different study groups, the benefits of liver resection have been accepted for selected patients with HCC harbouring PVTT and are implemented in their treatment guidelines [30–32]. Moreover, the recent management guidelines from the AASLD recognize that the definition of operability and resectability is quite heterogeneous and could differ significantly in clinical practice. A growing body of evidence shows a potential advantage of resection beyond early BCLC stages, so the role of strict treatment guidelines needs to be reconsidered among pioneers worldwide. In high-volume centres, different treatments are assigned to different groups of patients, creating a great overlap between recommended therapies and prognostic stages in daily clinical practice [14, 24, 33].
Mechanisms of PVTT formation
The mechanism of PVTT formation is not entirely understood and until recently has not been elucidated. Many factors are implicated in this process, with haemodynamic and biological factors playing an important role. Among researchers, the mechanical force seems to be of greater importance when compared to biological cancer cell factors to determine the metastatic route. Apart from the traditional belief that cancer cells directly infiltrate the venous wall and grow into the portal vein, researchers have identified PVTT distant from liver tumours, demonstrating that the mechanism is much more complex [34]. It has been advocated that a tumour microenvironment in cirrhotic patients plays a major role, and not only genetic or biological factors influence the pattern of vascular invasion. The complex mechanism of PVTT is associated with hepatic artery-portal fistula (HAPVF) and a portal vein counter current (PVCC) and this hypothesis has been demonstrated in PVTT patients before [35].
PVCC mechanisms of formation can be briefly summarized as follows: HCC nodules might block central veins and feeding arteries to the cancer nodules, which communicate with small portal branches. The high pressure creates a system with regional portal hypertension and increased pressure in sinusoids, which in turn causes HAPVF and PVCC. Tumour vessels transform into drainage channels, and cancer cells migrate intra-hepatically through these reversal blood flows. These cells are prone to implantation in the obstructed portal vein branches [36]. On the other hand, the biology of the tumour cell exhibits functions that are not entirely understood. This is probably the reason why HCC patients rarely present with splenic or hepatic vein thrombosis. Studies have shown that portal vein blood inhibits the apoptosis and promotes the migration and invasion of CSQT-2 cells. In addition, portal vein blood could up-regulate the expression of matrix metalloproteinase-2 (MMP-2), which is considered to be strongly associated with tumour metastasis [37]. Moreover, lower concentrations of IL-12 in portal vein serum could be linked with a negative effect on the apoptosis of PVTT-originated cells. The aforementioned molecular alterations suggest that the microenvironment of the portal venous system could enhance the infiltrative capacity of HCC cells. Additionally, the transformation of macrophages activated by the tumour environment and the release of several growth factors that could promote tumour metastasis need to be further studied [34, 35].
Classification of PVTT
In the presence of PVTT a careful selection is paramount, when curative aggressive invasive treatment is advocated. To be able to identify subgroups of patients who could benefit from surgery, a universal classification of PVTT is required. Various classification systems have been used by several centers especially in the East, where major complicated operations for advance stage HCC are more often performed. In this regard the Liver Cancer Study Group of Japan (LCSGJ) developed a macroscopic classification of HCC with PVTT: Vp0, no PVTT; Vp1, a PVTT distal to, but not in, the second-order branches of the portal vein; Vp2, PVTT in the second-order branches; Vp3, the presence of a PVTT in the first-order branches; and Vp4, the presence of a PVTT in the main portal vein or a contralateral portal vein branch or both [31]. For the first 2 stages surgical resection was deemed a feasible approach, while selected Vp3 or Vp4 patients could receive surgical resection with a 5-year survival of 18.3% [31, 38] (Table I). Further attempts to correlate overall survival and the stage of PVTT after liver resection have been proposed. Shi et al. have classified PVTT, known as Cheng’s classification, including stages from type I to type IV according to PVTT extension (Table II) [39]. The 1-, 2-, and 3-year OS rates were 54.8%, 33.9%, and 26.7% for type I patients, respectively. For type II the OS was 36.4%, 24.9%, and 16.9% respectively; 25.9%, 12.9%, and 3.7% for type 3 patients, respectively; and 11.1%, 0%, and 0%, respectively, for type 4 patients (p < 0.0001) [39]. Furthermore, Xu et al. have simplified the classification of PVTT into 2 groups: group A, with involvement of the main portal vein trunk or both the left and right portal veins, and Group B, with involvement only of the left or right portal vein [40]. The results regarding OS were 31.5% for group A after resection, while for group B they were 62.3%, 16.1%, and 5.2% in 1, 3, and 5 years. Similarly, Chen et al. divided PVTT patients into group A, with tumour thrombus located in the hepatic resection area or protruding into the first branch of the main portal vein beyond the resection line for < 1 cm, and group B, with PVTT extended into the main portal vein [41]. PVTT recurrence within 6 months after surgery in group B was significantly higher than that in group A: 76.9% vs. 11.3%, respectively. In addition, Fukumoto et al. divided macroscopically PVTT into “expansive” and “floating” type depending on how proximally or distally to the main portal trunk it has occurred and if the relative vessel maintains its original vascular calibre [22]. For example, in an expansive growth, the diameter of the portal vein becomes much larger than the calibre of the original one. This has implications in the surgical technique of liver resection and thrombectomy [22]. Most of the classifications offer the advantages of a relatively precise topographic staging in combination with ascending degree of severity. The surgical approach relies upon the type of PVTT, and so does the prognosis, which is determined from the extent of the thrombosis [42].
Table I
Classification status of PVTT in HCC according to current available systems
| Author [ref.] | Microscopic PVTT | Segmental branch | 2nd order PV branch | Left or right PV | Main PV | SMV |
|---|---|---|---|---|---|---|
| Shi [39] | I0 | I | II | III | IV | |
| Kudo [31] | Vp1 | Vp2 | Vp3 | Vp4 | ||
| Xu [40] | B | A (or both L/R PV | ||||
| Chen [41] | A | A (< 1 cm of resection line) | B (or > 1 cm of resection line) | |||
| Fukumoto [22] | Floating | Floating/expansive | Expansive | |||
Table II
Cheng’s classification of PVTT
Surgical efficacy vs. non-surgical treatments
Several modalities have been attempted to increase survival in HCC patients with PVTT [43]. In a Japanese nationwide survey, survival rates at 1, 3, and 5 years after initial diagnosis for the surgical group of patients was significantly higher compared to the non-surgical treatment group, with survival rates being 70.9%, 43.5%, and 32.9% vs. 62.9%, 31.6%, and 20.1%, respectively. Even if the surgical treatment has reached a survival benefit independently from other prognostic factors such as tumour size or aetiology, which was not significant in the advance stage of PVTT. Thus, liver resection was recommended when PVTT was limited to the first-order branch of the portal vein [12, 28, 31, 38]. In another large cohort study from China, the median survival time for type I and II patients were 15.9 and 12.5 months, respectively, with better results than non-surgical treatments [44]. Several other studies presented comparable results while suggesting that type IV patients are not qualified for surgery [18, 42, 45, 46]. Furthermore, attempts to compare TACE to surgical treatment showed better prognosis in the surgical group for type I/II, but not for type III/IV [44, 47–49]. A combination of preoperative TACE with surgery seems promising but failed to achieve a survival benefit for advance stage PVTT [15, 50–52]. Several existing meta-analyses including BCLC B patients have reported 5-year survival rates for surgery vs. TACE at 45% vs. 23%, respectively, and OS higher in liver resection than in TACE. Moreover, there has been no reliable study comparing resection or TACE with systemic target therapy for BCLC stage-C HCC patients. Therefore, surgery should be considered a therapeutic option tailored to a carefully selected group of BCLC stage-B HCC patients with well-preserved liver function [24, 53–55]. Another meta-analysis showed that HCC patients with branch type PVTT and surgery had better result in terms of prognosis, but showed no benefit over TACE or sorafenib in patients with main PVTT [56–59], whereas other published studies showed that TACE is associated with similar outcomes when treating patients with type III PVTT (p = 0.541) [60]. In Western countries, conversely, the first-line treatment option for HCC-PVTT is sorafenib, with median survival 10.7 months (6.5 months in Eastern countries) [46]. Even if the efficacy of sorafenib is not well established in PVTT patients, an OS of 8.1 months was demonstrated [61], while in an Asia-Pacific trial [62, 63], sorafenib was associated with modest prolongation of survival (5.6 vs. 4.1 months).
In addition, the effect of radiotherapy compared to surgery in a subgroup analysis showed that the 2-year OS in type I PVTT receiving 3D conformal radiotherapy (CRT) and surgery are 39% and 53%, respectively, (p < 0.001) indicating that surgery is superior to radiotherapy in terms of efficacy, while in type II PVTT the effect of both modalities was similar [64]. While modern radiotherapy, particularly in combination with other treatment options, may be feasible for HCC patients with PVTT, additional evidence is needed to confirm a survival benefit [63].
Surgical methods and techniques
Even though non-surgical treatment is recommended in HCC with PVTT patients, liver resection could be proposed in carefully selected cases based on the available scientific data. Decreased portal pressure after removal of the tumour thrombus might improve liver function and quality of life and potentially prolong survival [39, 57]. Taking into account data that report median survival for type I-IV PVTT of 6.2 to 64 months in patients who underwent resection and around 3 months for conservative treatments in type I–II, it seems reasonable that resection pathways might be the most promising option to follow [13, 65]. However, surgery due to technical challenges along with the underlying cirrhosis has been limited historically to patients with PVTT distally to the fist-order branch [63, 66–68].
Child status, extrahepatic spread, classification of PVTT, and total removal of thrombus are parameters that need to be carefully considered before offering aggressive surgical treatment [36, 44]. There is still some controversy regarding the theoretical advantage of anatomical resection to non-anatomical because anatomical resection can remove satellite lesions along the portal peripheral branches, but the significance has not been established in current practice. As a result, several surgical techniques have been introduced. Depending on the level of thrombus to the liver resection line, en bloc resection could be achieved. For example, for Cheng’s type I PVTT a segmentectomy could be performed, while a formal hemihepatectomy is indicated for a type II. On the other hand, if PVTT extends the resection line (type III/IV), hepatectomy and thrombectomy plus portal vein reconstruction is a suitable technique [44, 46].
Major liver resections, however, according to PVTT extension, could impair liver function, and for this reason en bloc resections are occasionally abandoned. In the so-called peeling off (PO) technique, the thrombus is removed from the internal wall of the portal veins along with sparing parenchyma tumour resection. In this manner portal vein reconstruction is not necessary, and the liver function could be maintained because lesser resections are applied. This approach is supported by the hypothesis that the risk of cancer spread could not be higher because the blood flow is already exposed to tumour cells and because tumour thrombus rarely infiltrates the portal vein wall [17]. Inoue et al. [17] presented satisfactory results with 3- and 5-year OS rates for the PO group of 46% and 39%, respectively, which is comparable with those of the en bloc group (41% and 41%). Excellent results have been presented regarding this type of thrombectomy, with 5-year survival rates in Vp3 and Vp4 of up to 21.2% and with no difference in terms of long-term outcomes [69]. Conversely, Zhang et al. [70] showed a significantly increased recurrence rate of vascular invasion when compared with the en bloc group (23.9% vs. 9.7%, respectively, p = 0.005).
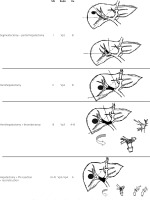
In addition, the back flow technique introduced by Fukumoto et al. [22], treating patients with contralateral first portal branch PVTT with crushing and suctioning using the back-flow pressure of the portal system, has been linked with 1-, 3-, and 5-year OS rates of 53.6, 15.3, and 7.7%, respectively. Ban et al. [69] improved the outcomes in type Vp3/Vp4 patients with the ‘thrombectomy first’ technique, presenting 1-, 3-, and 5-year survival rates of 69.6%, 37.4%, and 22.4%, respectively. Even if in the aforementioned studies the results regarding the recurrence at liver remnant, residual vein tumour, and disseminated peritoneal disease are promising, further studies are needed to confirm and justify these outcomes [16, 17, 27, 70, 71]. Based on the location and extent of PVTT, commonly used surgical methods are summarized in (Figure 1). As stated, surgery could be feasible and effective in advance stages of HCC and should be considered on a case-by-case basis. Clinicians should be aware of the disadvantages of various strategies and the relatively aggressive approach must be tailored to each patient [72–79].
Conclusions
Patients harbouring HCC with PVTT present more often with complications related to the degree of cirrhosis, and they have a worse prognosis. The accuracy of the classification of PVTT is paramount when aggressive surgical treatment has been anticipated. Careful appreciation of predictors that are associated with dismal prognosis is necessary before planning major resection. Combination treatment strategies as a feasible treatment modality should be performed after careful selection. Furthermore, the implementation of downstaging techniques could increase the pool of patients who could benefit from surgery afterwards. Despite the fact that PVTT is a major prognostic factor, efforts to improve prognosis in such patients rending the necessity to implement liver resection in future treatment guidelines must be seriously considered. Well-designed studies should focus on this issue, comparing surgery to other treatment strategies with the aim of improving outcomes, increasing the pool of patients to be treated, and reducing tumour recurrence. In the future, a combination of biomarkers, sophisticated imaging, and individualized treatment according to the extent of PVTT could add more than just an improvement in quality of life in these patients.