Introduction
Colorectal cancer is most common in developed countries. Each year, more than one million people develop colon cancer, and nearly 70,000 people die from this malignancy. Although medicine has made great strides in the treatment of colorectal cancer, the prognosis of patients is still poor. However, more than 10% of patients who are in stage IV survive for up to 5 years. It is difficult to find the main cause of colon cancer, so it is necessary to introduce new methods that will accurately diagnose the cause of this malignant disease [1, 2].
The transforming acidic coiled-coil 3 (TACC3) belongs to the TACC family of centrosome proteins and has a highly conserved C-terminal curly coil domain. TACC3 is a gene associated with cancer development. It regulates the formation of microtubules throughout the cell cycle [1–3]. A great number of studies have shown that TACC3 is highly expressed in gastric cancer, cholangiocarcinoma, multiple myeloma, osteosarcoma, prostate cancer, glioblastoma and non-small cell lung cancer, and breast cancer [3–12]. However, inhibition of TACC3 expression has been detected in ovarian and thyroid cancers [13, 14].
Aim
On the basis of this background, the present study applied immunohistochemical methods to investigate the TACC3 reactivity and established the model of clinicopathological factors so as to explore the connections of such factors and prognosis in colon adenocarcinoma patients.
Material and methods
Tissue samples
All colon adenocarcinoma samples (n = 97) were collected from patients who underwent radical surgery at the Municipal Hospital in Jaworzno. All patients were definitively diagnosed with colon adenocarcinoma and did not receive adjuvant chemotherapy, radiotherapy, or immunotherapy prior to surgery. The exclusion criteria were as follows: (1) history of previous malignant disease, (2) familial adenomatous polyposis, (3) inflammatory bowel disease, (4) preoperative anti-cancer treatment, and (5) evidence of distant metastasis. The clinicopathological characteristics obtained from the medical records were as follows: age, gender, location of tumour, grade of tumour differentiation, depth of invasion, regional lymph node involvement, operation record, treatment record, reoccurrence, and vital status at the last follow-up date.
The colon adenocarcinoma specimens belonged to 48 men and 49 women (mean age: 68; range: 33–89 years). Tumours were located in the proximal part of the colon in 50 (51.5%) cases and in the distal part of the colon in 47 (48.45%). Three levels of differentiation were used to classify the grading as follows: well differentiated (G1) 14 (14.43%) cases; moderately differentiated (G2) 52 (53.61%) cases; and poorly differentiated (G3) 31 (31.96%) cases (Table I).
Table I
Demographic, clinical, and tumour-related characteristics of patients included in the study (n = 97)
Immunohistochemical staining
For the immunohistochemical studies the paraffin-embedded specimens were cut into 4-µm-thick sections, fixed on Polysine slides, and deparaffinised in xylene and rehydrated through a graded series of alcohol. To retrieve the antigenicity, the tissue sections were treated twice with microwaves in a 10 mM citrate buffer (pH 6.0) for 8 min each. Subsequently, sections were incubated with rabbit polyclonal antibody to TACC3 (final dilution 1 : 600) (Novous Biologicals a Biotechne Brand cat. number NBP2-67671). For visualisation of protein expression, the sections were treated with the BrightVision detection system and Permanent AP Red Kit (Zytomed). Mayer’s haematoxylin was used to counterstain the nuclei.
The scores were assigned separately for the stained area and for the intensity of the immunohistochemical reaction. Quantification connected to the stained area of the tissue section was performed as follows: (1) < 33% of cells showed immunoreaction, (2) 33–66% of the cells had positive reaction to TACC3, and (3) > 66% of the cells were positive. The intensity of the immunohistochemical reaction was quantified as follows: (1) absent or weak, (2) moderate, or (3) strong. Each tissue section was characterised by a final grade derived from the multiplication of the stained area and the intensity of the staining. The TACC3 expression was considered to be absent/low for grade 1; moderate for grades 2, 3, and 4; and strong for grades 6 and 9.
Statistical analysis
Statistical analyses were conducted using Statistica 9.1 (StatSoft, Poland). The clinical characteristics of the patients in relation to TACC3 immunoreactivity were assessed by performing the Kruskal-Wallis test and U Mann-Whitney test. The Kaplan-Meier Method was used to study survival curves and the long-rank test to compute differences between the curves.
Results
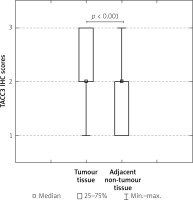
To investigate the prognostic function of TACC3 expression, the immunohistochemical analysis was performed in colon adenocarcinoma tissues and adjacent non-pathological samples in patients diagnosed with colon adenocarcinoma. It should be pointed out that expression of TACC3 protein in non-pathological samples was described as weak, moderate, or strong. In samples of colon adenocarcinoma, a high, moderate, and weak level of TACC3 protein was demonstrated (Figures 1, 2). Among the 97 colon adenocarcinoma samples, 24 (24.74%) showed weak immunohistochemical reaction, 27 (27.84%) demonstrated moderate immunoreactivity, and 46 (47.42%) revealed strong expression. The relationships between the TACC3 expression level and each clinicopathological parameter are summarised in Table II. As demonstrated, the level of the TACC3 immunohistochemical reactivity was not correlated with demographic factors including gender and age. Correlations were also not observed between TACC3 immunohistochemical reaction and clinicopathological factors (all p > 0.05; Table II).
Figure 1
Immunohistochemical expression of TACC3 protein in tumour tissue: A, B – control, C, D – weak expression of TACC3 protein in colon adenocarcinoma samples, E, F – moderate expression of TACC3, G, H – strong expression of TACC3 (C, E, G – 25×; D, F, H – 100×)
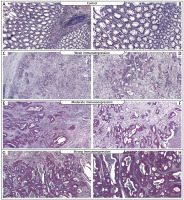
Table II
Corelations between TACC3 immunoexpression and clinicopathological characteristics in colorectal cancer patients
The Kaplan-Meier survival analysis showed that the overall survival rate in the group of patients with a low expression level of TACC3 was not significantly longer than that for patients with a moderate or strong level of Notch3 immunoreactivity (p < 0.001; Figure 3). The TACC3-low patients had an average survival time of 39.3 months (95% CI: 31.300–47.283), whereas the TACC3-moderate groups had an average survival time of 37 months (95% CI: 30.077–43.775). The TACC3-strong patients had an average survival time of 35.5 months (95% CI: 33.134–40.536). The average survival time for all the patients was 36.8 months (95% CI: 33.134–40.536). Interestingly, in the univariate analysis the grade of tumour differentiation and TACC3 immunoexpression in non-pathological samples were found to be significantly correlated with reduced 5-year survival. Moreover, a multivariate analysis demonstrated that the grade of tumour differentiation (HR = 2.740; 95% CI: 1.864–4.027, p < 0.001) and TACC3 immunoexpression in healthy ones (HR = 1.700; 95% CI: 1.073–2.694) were independent risk factors for worse survival. The results of the univariate and multivariate analyses for OS are given in Table III.
Figure 3
Kaplan-Meier survival curves of colon adenocarcinoma patients with weak, moderate, and strong expression of TACC3 protein; follow-up period = 60 months
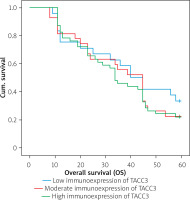
Table III
Univariate and multivariate analyses of various prognostic parameters in colon adenocarcinoma patients using Cox regression analyses
Discussion
TACC3 has been characterized as a gene associated with cancerogenesis. Through the changing cellular processes, initiating oncogenic signal transduction pathways and inducing genomic instability, the up- and downregulation of TACC3 is clearly related to cancer development. Most studies have demonstrated that TACC3 expression is upregulated in many cancers. However, inhibition of TACC3 expression has been detected as well [15]. It seems therefore that TACC3 plays different roles in different types of cancer [2–15].
The results of our study demonstrated that expression of TACC3 was up-regulated in colon adenocarcinoma tissue in comparison to healthy ones. Interestingly, the level of TACC3 immunohistochemical expression was not correlated with clinicopathological factors and cancer progression. Moreover, the expression of TACC3 in colon adenocarcinoma patients with lymph node metastasis was not significantly higher than that observed in patients without metastasis. The level of expression was not correlated with grade of histological differentiation as well. However, in patients with G3 adenocarcinoma we did not observe the low level of immunoreactivity. In those patients we detected moderate and strong levels of immunohistochemical reaction. Importantly, the level of TACC3 expression in cancer samples was not correlated with the overall survival of patients. It should be pointed out that the level TACC3 expression in healthy tissues was correlated with a reduced level of survival; apart from the feature connected with the level of histological differentiation, it was the second factor connected with poor prognosis. In contrast, Du et al. revealed that high expression of TACC3 in colon samples can reduce the prognosis of patients diagnosed with this malignancy [16]. In gastric cancer patients, the expanded expression of TACC3 played an important role in extracapsular infiltration, which could be used as an independent predictor of shorter OS [4].
As mentioned earlier, some types of tumour show reduced levels of TACC3. Ulisse et al. showed that the expression of this protein was reduced in various thyroid cancer-derived cell lines, while it was not reduced in a benign thyroid follicular tumour cell line. In particular, the reduction of TACC3 expression played a very important role in 2 cell lines that originated from the highly aggressive human anaplastic thyroid carcinoma [14]. There was an increase and a decrease in regulation in differentiated thyroid cancer (DTC) tissue samples. 56% of the DTCs showed reduced TACC3 gene expression. In contrast, TACC3 expression was increased by 44% [14]. In thyroid cells, the TACC3 protein can act as both a transforming agent and a tumour-fighting agent, as demonstrated in other cell types.
In a multifaced genomic evaluation, TACC3 was established as a potential oncogene. For example, blocking TACC3 with KHS101 in breast cancer cell lines inhibited cell viability, motility, EMT, and breast cancer stem cells and induced apoptotic cell death under in vitro conditions. The reduction of the TACC3 level in ESCC blocked the ability to migrate ESCC cells. From this it can be concluded that TACC3 may be a modulator in the surveillance of metastasis. The reduction of the mesenchymal marker vimentin and the increase of the epithelial markers E-cadherin and ZO-1 have demonstrated the importance of TACC3 in the physiology of epithelial-mesenchymal transition [17]. TACC3 deficiency inhibited the epithelial-mesenchymal transition phenotype by activating the PI3K/Akt and ERK signalling pathway. It seems, therefore, that TACC3 can grow tumours by increasing cell proliferation, the population of cancer stem cells, and the migration of cancer cells.