Introduction
Crohn’s disease is a chronic inflammatory disorder that is relapsing in nature. Progression from inflammation to penetrating disease occurs following fibrosis. The aim of therapeutic strategies is to rapidly achieve and maintain remission, with the ultimate goal of improving the long-term prognosis [1]. In addition, emphasis is re-focused from the control of symptoms and normalisation of biochemical markers to the achievement of complete mucosal healing [2]. Importantly, the absence of clinical symptoms does not exclude active disease [3].
Conventional endoscopy with biopsy is the reference standard for diagnosing Crohn’s activity, and it remains key for the therapeutic management of Crohn’s disease [4]. However, it is an invasive technique, and its use beyond the colon and the final few centimetres of the ileum presents technical difficulties. Moreover, biopsy offers limited information about transmural and extra-enteric disease patterns. Colonic strictures represent an additional challenge associated with the biopsy procedure [5]. Due to its time-course, subjects suffering from Crohn’s disease often undergo multiple imaging exams to evaluate symptom recurrence. Magnetic resonance imaging (MRI) is increasingly recognised as a radiological modality of choice due to its lack of exposure to ionising radiation and established accuracy for detecting active disease [6]. In particular, the combination of MRI enterography (MRE) with diffusion-weighted imaging (DWI) of restricted areas may enhance the accuracy in determining Crohn’s disease activity compared to the use of either technique alone [7].
Visualisation of layered bowel wall enhancement (with visible dissections) can be obtained through dynamic examination following intravenous administration of a contrast medium [8, 9]. The apparent diffusion coefficient (ADC), a biophysical parameter representing the rate of diffusion, is also altered in inflamed regions [10, 11].
We hypothesised that, based on MRE layered bowel wall enhancement and ADC, measured in the affected parts of the intestine, it is possible to effectively differentiate between active and chronic phases of Crohn’s disease.
Aim
The aim of this study was to create a multidimensional diagnostic model that enables the differentiation of Crohn’s disease phases.
Material and methods
This study involved 125 patients: 55 women (aged 19 to 66 years) and 70 men (aged 12 to 67 years). All patients were referred for participation in this study based on clinical symptoms suggestive of Crohn’s disease. All patients in the study underwent MRE and ADC measurements for the first time.
The inclusion criteria were as follows: clinical suspicion of Crohn’s disease posed by a gastroenterologist based on clinical symptoms, no previous treatment for Crohn’s disease, and no contraindications for MRI examination or for use of an intravenous paramagnetic contrast agent. The exclusion criteria were as follows: lack of clinical suspicion of Crohn’s disease, diagnosed and previously treated for Crohn’s disease, morphological MRI features of bowel cancer, and contraindications to MRI examination.
The MRE examination was performed with a 3T Achieva scanner (Philips, Eindhoven, Netherlands). Patients were given 2 Scopolan tablets an hour before the test as well as a contrast solution consisting of 750 ml of water and 250 ml of mannitol to drink within 1 h. During the first phase of the study, the test was performed in the coronal and transverse planes. T1- and T2-weighted images were obtained in the TSE (Turbo Spin Echo), TFE (Turbo Field Echo), TFE/SPIR (Turbo Field Echo/Spectral Presaturation with Inversion Recovery), BTFE (Balanced Turbo Field Echo), and DWI sequences. During the second stage, an intravenous paramagnetic contrast agent was administered by a manual bolus through a cannula inserted into the cubital vein. The injection was terminated with 30 ml of saline. Following administration of the contrast agent, images were obtained using the THRIVE (T1-weighted High Resolution Isotropic Volume Examination) and TFE/SPIR sequences in the coronal and transverse planes. ProHance (Gadoteridol, Bracco Diagnostics, Milan, Italy) was used for all examinations.
Two radiologists analysed the acquired images for traits characteristic of changes during Crohn’s disease. The result was obtained by consensus.
The number of occupied regions was determined in patients who showed changes characteristic of Crohn’s disease. The considered regions included the jejunum, ileum, ileocecal region, caecum, colon, sigmoid colon, and rectum. A total of 125 occupied regions were found, which were then analysed. The following radiological features were analysed: thickness of the occupied section, length of the occupied section, number of lymph nodes present, layered bowel wall enhancement, total transitions on fat tissue, features of restricted diffusion in the DWI, and ADC values (Figures 1–4).
Figure 1
Non-fat saturation T2-weighted coronal image showing significant bowel wall thickening and luminal narrowing of the terminal ileum (arrowhead)
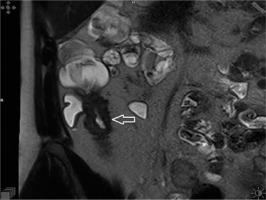
Figure 2
T1-weighted post-contrast coronal image showing stratification of the intestinal wall with trilaminar aspect of the bowel wall enhancement (arrowhead)
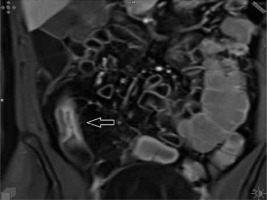
Figure 3
Diffusion-weighted (b = 800 s/mm2) axial image demonstrating high signal intensity due to restricted mural diffusion (arrowhead)
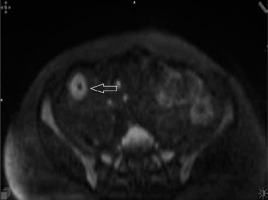
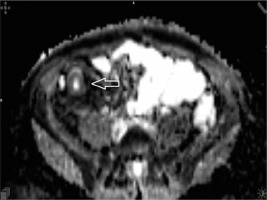
Figure 4
Apparent diffusion coefficient (value 1192) axial image demonstrating low signal intensity on the apparent diffusion map due to restricted mural diffusion of the terminal ileum (arrowhead)
Definitive diagnosis of Crohn’s disease was based on histological examination of material collected during ileocolonoscopy (96 patients), surgery with subsequent histopathological examination (17 patients), and capsule endoscopy (12 patients).
Results
The final discrimination model was based on 2 variables: ADC and layered bowel wall enhancement. The stepwise analysis revealed that the best set of explanatory variables (Table I) was obtained by one of the simplest models (2-dimensional) among all considered. This model was statistically significant (p < 0.001), as well as each of its variables. All main assumptions were also fulfilled. The best variable for discriminating between active and chronic phases of Crohn’s disease was ADC (Wilks’ λ = 0.724, p < 0.001). The classification matrix (Table II) based on classification function (Table III) was able to identify the active form of Crohn’s disease with a probability of 93.06% and the chronic form with a probability of 75.57%, which gave a total predictive value of 85.60%.
Table I
Results of stepwise analysis for the best set of explanatory variables
| Label | Variable | Wilks’ λ = 0.554, F (2,122) = 49.143, p < 0.001 | ||||
|---|---|---|---|---|---|---|
| Partial Wilks’ | F | P-value | Toler. | 1-Toler (R2) | ||
| A | ADC | 0.724 | 46.586 | < 0.001 | 0.953 | 0.047 |
| W | Layered bowel wall enhancement | 0.917 | 11.047 | 0.001 | 0.953 | 0.047 |
Discussion
Numerous studies have evaluated the use of MRE and DWI as potential markers of the grade and stage of Crohn’s disease [12–14]. However, to the best of our knowledge, this is the first study performed on a large group of subjects to determine that the combination of MRE layered bowel wall enhancement and ADC value may yield a high predictive value, particularly with respect to discriminating the active form of Crohn’s disease from the chronic form.
A study by Koh et al. [8], which compared MRE with histopathological examination, showed diffuse bowel wall enhancement in 17 segments and a layered pattern in only 7 segments. The authors demonstrated that the intravenous administration of gadolinium results in significantly higher signal intensities in actively inflamed segments than those of bowel loops that appear normal. In this study, a ratio of 1.3 : 1 or more had a sensitivity of 68% and a specificity of 94% for active disease on a per-segment basis. Importantly, the authors analysed diffuse and layered bowel wall enhancement. Layered enhancement was seen only in patients with active phase of disease.
Bowel wall enhancement in active Crohn’s disease was also reported by Macarini et al. [15]. However, this study did not report direct correlations with histopathological changes, nor did it state how many segments were analysed. Research by Lasocki et al. [16] confirmed the high specificity and predictive value of MRE layered bowel enhancement in a group of 26 subjects with Crohn’s disease. Compared to previous studies using MRE layered bowel wall enhancement, our predictive model was based on a large group consisting of 125 subjects with Crohn’s disease.
Oto et al. [10] and Kiryu et al. [17] were the first to assess ADC as a radiological marker of active Crohn’s disease. In the study by Oto et al. [10], of the 19 inflamed segments, ADC detected 18 (grading score of 2 or 3), thus yielding a sensitivity of 94.7%. Kiryu et al. [17] analysed a total of 188 segments from 31 patients (17 with active Crohn’s disease), in which ADC showed high specificity (81.4%) and sensitivity (86.0%) as a marker of active Crohn’s disease. Restricted diffusion in inflamed bowel segments was later investigated by several other authors; however, these studies included a limited number of patients [12–14].
The main advantage of DWI protocols is the avoidance of gadolinium-based contrast medium. Inflamed tissue restricts the diffusion of water, leading to a high DWI signal density, when the normal tissue signal is suppressed. Consequently, DWI with high b-values possess a spontaneous contrast that may highlight bowel segments with active disease in a similar manner to contrast-enhanced images. Therefore, DWI-based techniques are destined to replace classic contrast-enhanced MRE imaging techniques [18].
The strength of our model is its good ability to discriminate between active and chronic Crohn’s disease. It is sometimes challenging to differentiate fibrotic lesions and active inflammation on MRE images, particularly when these 2 pathologies coexist and the MRE features overlap. In active inflammation, the wall of the bowel exhibits restricted diffusion, with a low ADC value and high signal intensity on DWI, assessed using low and high b-values, respectively. Fibrotic tissue does not restrict diffusion, and it shows a higher ADC value and a signal that diminishes at high b-values.
Due to several shortcomings of DWI/ADC models, we propose a mixed diagnostic schedule that combines MRE and ADC. Using 2 parameters, namely MRE layered bowel wall enhancement and ADC, we were able to overcome the major shortcomings of both MRI techniques.
Importantly, our model does not require the establishment of cut-off values for ADC. The measured ADC (A) value was simply added to the equation, with active Crohn’s disease defined as –6.339 + 4.747 × W + 0.008 × A, while chronic Crohn’s disease was defined as –11.365 + 2.812 × W + 0.012 × A. The MRE layered bowel wall enhancement (W) was entered into the equation as “1” if at least one stratification of the intestinal wall was present, otherwise “0” was used. Therefore, our model eliminates a significant amount of subjectivity in the assessment of Crohn’s disease.










