Introduction
Functional dyspepsia is one of the common diseases of the gastrointestinal tract. The basis for its diagnosis is the exclusion of organic gastric and duodenal diseases as well as inflammatory, metabolic and psychiatric diseases. The pathogenesis of dyspepsia is complex and not well understood. Pathogenetic factors include gastric secretory and myoelectrical dysfunction, gut-brain axis dysregulation, psycho-emotional disorders and other factors [1, 2]. Helicobacter pylori infection may be one of the causes of dyspeptic symptoms [3]. However, the course of the infection is known to be asymptomatic in most patients. On the other hand, eradication of this bacterium does not always eliminate the ailments [4]. Symptomatic treatment using agents that modulate gastric secretion and motility also does not result in lasting improvement [5]. Therefore, in this disease there should be considered involvement of other pathogenetic factors, including intestinal bacteria. A significant effect of dysbiosis has been documented in other functional bowel diseases [6, 7]. Less attention has been paid to the role of intestinal microbiome changes in the pathogenesis of functional dyspepsia [8]. However, attention was paid to frequent occurrence of small intestinal bacterial overgrowth in this group of patients [9, 10], but the results were not related to the clinical type and severity of dyspeptic complaints.
Aim
The aim of the study was to evaluate the results of the hydrogen breath test in relation to the clinical picture of functional dyspepsia.
Material and methods
The study included 196 patients, aged 24–68 years, divided into three groups. Group I (n = 40) – healthy subjects; group II (n = 72) – patients with functional dyspepsia in the form of postprandial distress syndrome (PDS); group III (n = 84) – patients with functional dyspepsia in the form of epigastric pain syndrome (EPS).
The diagnosis of functional dyspepsia was based on Rome IV Criteria [11].
The study included only patients whose dyspeptic complaints were of a chronic nature, were not treated before or the medications were ineffective.
In the PDS group, postprandial pain was accompanied by a feeling of early fullness, nausea and abdominal bloating.
In the EPS group, epigastric pain was of hunger and/or night nature and was accompanied by heartburn and acid reflux.
Persons infected with H. pylori, with other gastrointestinal diseases, allergy, food intolerance, metabolic and mental diseases were excluded from the study.
In order to exclude concomitant diseases, upper and lower gastrointestinal tract endoscopic examinations with histological examination of the mucosa and the following laboratory tests were performed: blood count, bilirubin, alanine and aspartate aminotransferase, gamma-glutamyltranspeptidase, amylase, lipase, glucose, cholesterol, triglycerides, acute phase proteins and fecal calprotectin.
In the diagnosis of H. pylori infection, the urea breath test was performed using 75 g of 13C-labeled urea and a FANci-2 analyzer (Fisher Analyzer Instrumente GmbH).
The hydrogen breath test was performed using 10 g of lactulose and a Bedfont Gastrolyzer analyzer. The result confirming small intestinal bacterial overgrowth was assumed to be an increase in hydrogen concentration by more than 20 ppm compared to the baseline value within 90 min in accordance with currently accepted criteria [12].
Both breath tests were performed in the same week, after a previous 4-week ban on taking antibiotics, probiotics, prokinetics and gastric acid secretion inhibiting drugs. Moreover, a day before the examinations, the patients were not allowed to consume slowly absorbed carbohydrates (bread, potatoes) and fiber preparations.
Patients with a positive LHBT test indicating small intestinal bacterial overgrowth were assigned to antibiotic therapy. Rifaximin at the daily dose of 1200 mg was administered for 14 days without probiotic supplementation and 6 weeks after the end of antibiotic treatment the severity of dyspeptic symptoms was assessed using a 10-point visual scale and the LHBT test was repeated.
All patients were acquainted with the purpose of the study and gave their written consent to perform it. The approval of the Bioethics Committee of the Medical University of Lodz was obtained (RNN/179/14KB). The study was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice (GCP).
Statistical analysis
The Kruskal-Wallis test was used to compare the mean values and the Dunnett test and the χ2 test were used to compare the groups. The results were considered significant at p < 0.05. The calculations were performed with Statistica 9.1 (StatSoft, Inc, USA and MS Excel (Microsoft Co, USA).
Results
General characteristics of subjects included in the study are presented in Table I.
Table I
General characteristics of subjects included in the study: healthy subjects (control group), patients with postprandial distress syndrome (PDS), patients with epigastric pain syndrome (EPS)
In the group of 40 healthy subjects, in 38 (95.0%) the hydrogen test (LHBT) was negative, and in 2 of them the increase in the concentration of hydrogen in the expired air slightly exceeded the normal value and was 22 ppm and 24 ppm, respectively.
In the group of 72 patients with postprandial syndrome (PDS), a strongly positive hydrogen test was found in 35 (48.6%), and a positive correlation between the results of the LHBT test and the severity of dyspeptic symptoms (r = 0.838, p < 0.001).
In the group of 84 patients with epigastric pain syndrome (EPS), a positive LHBT test was found in 40 (47.6%) patients; differences between the groups were not statistically significant (p > 0.05). In this group, the increase in hydrogen concentration was faster and in 16 patients it exceeded normal values already within the first 60 min. However, the correlation between the LHBT test results and the severity of dyspeptic symptoms was also strongly expressed (r = 0.546, p < 0.01).
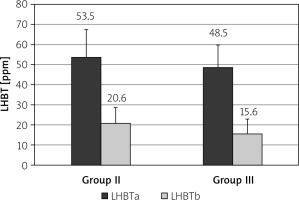
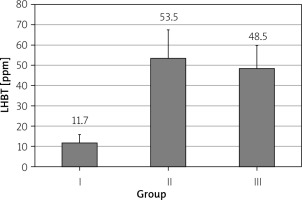
The mean positive LHBT test result in patients with PDS was 53.5 ±14.0 ppm and in patients with EPS 48.5 ±11.3 ppm (p > 0.05, Figure 1).
Figure 1
Results of lactulose hydrogen breath test (LHBT) in healthy subjects (group I), in patients with postprandial distress syndrome (group II) and in patients with epigastric pain syndrome (group III); differences between all groups statistically not significant – p > 0.05
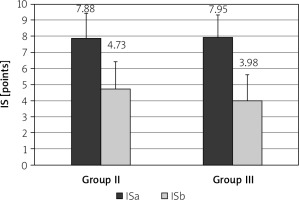
After treatment with rifaximin, the mean LHBT result decreased in the PDS group from 53.5 ±14.0 ppm to 20.6 ±7.99 ppm (p < 0.001) and in the EPS group from 48.5 ±11 ppm to 15.6 ±7.27 ppm (Figure 2, p < 0.001). Severity of dyspeptic complaints also decreased in the PDS group from 7.88 ±1.54 points to 4.73 ±1.69 points (p < 0.001), and in the EPS group from 7.95 ±1.38 points to 3.98 ±1.64 points (p < 0.001, Figure 3). In particular, in 16 (22.2%) patients in the PDS group and in 12 (14.2%) patients in the EPS group the symptoms resolved completely.
Discussion
The obtained results indicate that gastrointestinal dysbiosis in the form of small intestinal bacterial overgrowth may be one of the causes of chronic dyspepsia. This relationship should be considered after excluding other causes of the ailments. The LHBT test may be useful in the diagnosis of functional dyspepsia, but its limitations should be taken into account. Positive test results also occur in patients without gastrointestinal complaints [13], which is confirmed by our observations.
The clinical picture is also the basis for the diagnosis of small intestinal bacterial overgrowth. This syndrome is usually manifested by altered bowel habits which are accompanied by abdominal pain and bloating and therefore cause suspicion of irritable bowel syndrome (IBS). Most researchers emphasize frequent coexistence of IBS and SIBO [14–17]. Others, however, question the specificity of LHBT and believe that it does not serve to differentiate between the two syndromes [18, 19]. The relationship between SIBO and functional dyspepsia may be even more controversial. Costa et al. [20] examined 23 patients with FD and in 13 (56.5%) of them obtained a positive LHBT result. Similarly, Petzold et al. [10] found a positive LHBT result in 44.4% of patients with functional dyspepsia. Del Zompo [21] suggested that H. pylori infection alters the composition of the intestinal microbiota and facilitates the production of methane in the gastrointestinal tract. Some researchers believe that treatment with proton pump inhibitors (PPIs) promotes the development of SIBO, but others question this causal relationship [22]. In turn, Schindler et al. [19] showed no significant differences in LHBT results obtained in 146 patients with functional dyspepsia and 50 healthy volunteers. However, it should be emphasized that in most of the aforementioned studies LHBT results were not analyzed in detail with respect to the clinical picture of dyspepsia. Moreover, the discrepancy in the results obtained by different researchers may be due to a number of reasons, such as the heterogeneity of the material, differences in the doses of the used lactulose and different criteria for assessing the obtained results [13, 22, 23]. In our own research we adopted the current criteria of the American consensus, according to which a positive LHBT test diagnosing SIBO was considered if a rise in breath hydrogen was 20 ppm above basal levels within 90 min after ingestion of 10 g of lactulose. Nevertheless, a detailed analysis of the results showed individual differences, with different initial concentration and different dynamics of the increase of hydrogen concentration in the expired air. Within 90 min both its rapid rise and flatter curves were observed. Furthermore, no significant correlation was found between LHBT test results and intensity of clinical symptoms. Thus, it can be assumed that not only quantitative but also qualitative changes in the intestinal microbiome are responsible for dyspeptic symptoms. In the process of digestion and metabolism of nutrients, many biological compounds which exert a variety of effects on gastrointestinal function are secreted [24, 25]. An example of this is the metabolism of tryptophan, which is a substrate for many compounds of the serotonin and kynurenine pathway. The expression of the basic enzymes of these pathways, i.e. tryptophan hydroxylase (TPH-1) and indoleamine 2,3-dioxygenase (IDO-1), are significantly influenced by intestinal bacteria [26, 27]. Moreover, some bacterial strains have the ability to synthesize serotonin [28], dopamine [29] and probably other compounds that affect the motor and secretory function of the gastrointestinal tract and visceral sensitivity. The action of these neurotransmitters mainly occurs at the site of their release but they are also partly absorbed into the peripheral blood and may affect the functions of many organs. It can be assumed that greater insight into the gut microbiome can explain the variety of symptoms of functional gastrointestinal diseases, including dyspeptic symptoms. This is supported by the beneficial effect of antibiotic therapy in patients with functional dyspepsia.