Introduction
Gastroesophageal reflux disease (GERD) results when gastric contents reflux into the oesophagus; it is often accompanied by symptoms such as heartburn, acid regurgitation, and dysphagia. Although GERD itself is not fatal, its symptoms affect health-related quality of life and work productivity. Without effective treatment, serious complications such as oesophageal stricture, ulceration, or Barrett’s oesophagus may develop [1].
Even at low doses, aspirin can cause gastrointestinal mucosal injury by inhibiting prostaglandin biosynthesis. However, low-dose aspirin (LDA) discontinuation can increase the risk of cardiovascular events for patients with a clear indication [2]. The same may occur with non-steroidal anti-inflammatory drugs (NSAIDs), commonly used to manage pain or inflammatory symptoms in chronic conditions. Although NSAIDs can cause gastrointestinal mucosal injury, their discontinuation is not always feasible because pain may recur and negatively impact the quality of life [3]. Therefore, it is clinically important to prevent mucosal injury while continuing LDA or NSAID therapy.
Gastric acid secretory inhibitors effectively treat GERD, peptic ulcer disease, and Helicobacter pylori infection and prevent LDA- or NSAID-induced peptic ulcers [4]. Proton pump inhibitors (PPIs), released in the late 1980s, dramatically improved gastric acid-related conditions [4]. Nowadays, potassium-competitive acid blockers (P-CABs) have emerged to promote a better antisecretory effect addressing these unmet needs associated with acid-related disease management, including but not limited to the treatment of advanced-grade erosive oesophagitis, refractory GERD, and erosive oesophagitis maintenance treatment [5, 6].
The first P-CAB used in clinical practice was revaprazan, available in South Korea and India since 2007. Despite the early effect on acid suppression by revaprazan, there are no reports that revaprazan is more effective than PPIs for other gastric acid-related conditions [4]. In 2015, vonoprazan became available in Japan for the treatment of gastric ulcer, duodenal ulcer, and erosive oesophagitis, and to prevent LDA- or NSAID-induced ulcer recurrence [7, 8].
Aim
We conducted a systematic review to assess whether vonoprazan effectively treats patients diagnosed with GERD oesophagitis or with peptic ulcers induced by chronic use of aspirin or NSAIDs.
Material and methods
Study design
A systematic review of the current literature was conducted to answer the proposed objectives, using the PICO model (population; intervention; control; outcome). Thus, we searched for studies that evaluated whether vonoprazan effectively treats patients diagnosed with GERD oesophagitis or with peptic ulcers induced by chronic use of aspirin or NSAIDs.
Search strategy
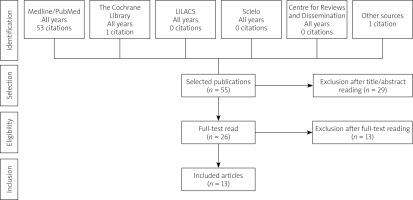
A literature search was conducted through 22 April 2021, using Medline via PubMed, Cochrane Library, Lilacs, Scielo, and Centre for Reviews and Dissemination (CRD) electronic databases for the terms in Table I. Additionally, manual searches of bibliographic references and abstracts of selected publications complemented electronic searches.
Table I
Search queries and retrieval numbers
Eligibility and inclusion and exclusion criteria
Studies to be considered eligible should meet the following inclusion criteria: meta-analyses, systematic reviews, and phase III or IV randomized controlled trials (RCTs) or observational studies; studies involving patients using vonoprazan to treat GERD esophagitis or those in chronic use of aspirin or NSAIDs; analysis with potassium pump inhibitors as a comparator or without comparator and efficacy endpoints trials. Furthermore, we excluded articles under at least one of the following conditions: narrative review, guidelines, consensus articles, editorials, case reports, or case series; studies involving patients with Helicobacter pylori; studies using animal models; articles published in languages other than English, Portuguese, and Spanish.
Results
Study selection
Studies were selected after removing duplicate records, and we retrieved 55 titles. After applying the eligibility criteria, 2 reviewers selected 26 studies for a full reading. Of these, 13 were chosen and included in this review (Figure 1).
Studies characteristics
Of the 13 articles included in this qualitative analysis, 4 were prospective cohort studies, 1 was a follow-up study of a preceding prospective study, 1 was a retrospective cohort study, and 7 were randomized clinical trials. The number of patients ranged from 16 to 732, totalling 3703 patients included in the selected studies. A list of the included studies and the description of their general characteristics according to the comparator type are presented in Table II.
Table II
Characteristics of selected studies and clinical profile of patients
| Author, year [ref.] | Local | Population | Intervention | Comparator | Efficacy endpoints |
|---|---|---|---|---|---|
| Mizuno, 2020 [15] | Japan | 50 patients aged ≥ 20 years, with RE refractory to PPIs who had no endoscopic evidence of erosive esophagitis after the administration of VPZ 20 mg OD/4 weeks | Vonoprazan 10 mg OD/48 weeks (maintenance) | – | |
| Mizuno, 2018 [6] | Japan | 52 patients aged ≥ 20 years, with RE refractory to PPIs who had no endoscopic evidence of erosive esophagitis after the administration of VPZ 20 mg OD/4 weeks | Vonoprazan 10mg OD/24 weeks (maintenance) | – | |
| Umezawa, 2018 [17] | Japan | 29 patients with mild RE receiving maintenance therapy with PPIs | Vonoprazan 20 mg OD/24 weeks (on-demand) | – | |
| Tanabe, 2019 [16] | Japan | 16 patients with RE refractory to PPIs | Vonoprazan 20 mg OD/4 weeks and vonoprazan 10 mg OD/8 weeks (maintenance) | – | |
| Hoshino, 2017 [2] | Japan | 24 patients with PPI-resistant RE | Vonoprazan 20 mg OD/4 weeks and vonoprazan 10 mg OD/8 weeks (maintenance) | – | |
| Ochiai, 2021 [3] | United States | 30 patients with PPI-resistant RE | Vonoprazan 20 mg OD | Lansoprazole 30 mg OD Esomeprazole 40 mg OD Rabeprazole 20 mg OD | |
| Xiao, 2020 [11] | Asia | 468 patients with endoscopically confirmed EE | Vonoprazan 20 mg OD up to 8 weeks | Lansoprazole 30 mg OD up to 8 weeks | |
| Ashida, 2018 [18] | Japan | 607 patients ≥ 20 years, who presented with endoscopically-confirmed healed EE after vonoprazan 20mg OD/up to 8 weeks | Vonoprazan 20 mg OD/24 weeks (maintenance) | Lansoprazole 15 mg OD/24 weeks (maintenance) | |
| Kawai, 2018 [19] | Japan | 621 patients (439 in extension) with long-term LDA-associated peptic ulcers | Vonoprazan 10 mg OD or vonoprazan 20 mg OD/24 weeks (double blind) and ≤ 2 years (extension) | Lansoprazole 15 mg OD/24 weeks (double blind) and ≤ 2 years (extension) | |
| Mizokami, 2018 [20] | Japan | 642 patients receiving long-term NSAID therapy who are at risk of peptic ulcers recurrence | Vonoprazan 10 mg OD or vonoprazan 20 mg OD/24 weeks (double-blind) and 28–80 weeks (extension) | Lansoprazole 15 mg OD/24 weeks (double-blind) and 28–80 weeks (extension) | |
| Oshima, 2019 [14] | Japan | 32 patients ≥ 20 years with endoscopically confirmed EE and a recent history of at least weekly heartburn episodes | Vonoprazan 20 mg OD/14 days | Lansoprazole 30 mg OD/14 days | |
| Ashida, 2016 [13] | Japan | 401 patients (305 in extension) with endoscopically confirmed EE | Vonoprazan 20 mg OD/8 weeks (comparison) and vonoprazan 10 mg or 20 mg OD/52 weeks (maintenance) | Lansoprazole 30 mg/8 weeks | |
| Ashida, 2015 [12] | Japan | 732 patients ≥ 20 years with endoscopically confirmed EE | Vonoprazan 5 mg, vonoprazan 10 mg, vonoprazan 20 mg or vonoprazan 40 mg OD/8weeks | Lansoprazole 30 mg OD/8 weeks |
Efficacy of initial therapy
In a prospective study, oesophageal mucosal breaks were successfully treated by vonoprazan 20 mg once daily for 4 weeks in 21 (87.5%) out of 24 patients with PPI-resistant reflux oesophagitis [2]. In 3 comparative studies, vonoprazan 20 mg demonstrated non-inferior efficacy versus lansoprazole 30 mg in terms of erosive esophagitis healing rate at 8 weeks in a population with erosive oesophagitis [11–13].
In patients with endoscopically confirmed GERD, heartburn was relieved sooner with vonoprazan 20 mg than with lansoprazole 30 mg (p < 0.05). Heartburn was relieved entirely in 31.3% and 12.5% of patients on day 1 with vonoprazan and lansoprazole, respectively. Significantly more patients achieved complete nocturnal heartburn relief with vonoprazan than with lansoprazole (p < 0.01) [14].
In a retrospective study, the efficacy of vonoprazan on PPI-resistant refractory reflux oesophagitis after oesophagectomy with gastric pull-up was evaluated. A 20 mg/day dose of vonoprazan significantly improved mucosal breaks in 81.3% of the treated patients compared to patients who continued PPI use (14.3%, p < 0.001). Additionally, vonoprazan aided mucosal healing in 68.8% of the patients compared to continued PPI use (7.1%, p = 0.001). Vonoprazan 20 mg could improve mucosal breaks in patients with refractory reflux esophagitis who had undergone esophagectomy with gastric tube reconstruction [3].
Efficacy of maintenance therapy
In 5 open-label prospective studies, vonoprazan 10 mg once daily showed effective 8-week [11], 24-week [6], 48-week [15], and 52-week [13, 16] maintenance therapy of healed reflux oesophagitis or erosive oesophagitis refractory to PPIs [6, 11, 13, 15, 16]. On-demand therapy using 20-mg vonoprazan tablets is also an effective alternative maintenance therapy for mild reflux esophagitis [17].
The non-inferiority of vonoprazan 10 and 20 mg to lansoprazole 15 mg as maintenance therapy for patients with healed erosive oesophagitis was confirmed in a study that compared lansoprazole 15 mg, vonoprazan 10 mg, and vonoprazan 20 mg [18].
GERD symptoms
The median frequency scale for the symptoms of gastroesophageal reflux disease (FSSG) score was significantly lower on days 1–7, 14, and 28 after the initiation of vonoprazan than before its administration in prospective studies with no comparator [2]. A comparative study showed total FSSG scores were significantly improved on days 7 and 14 by vonoprazan and on day 14 by lansoprazole [14]. At the end of the 24-week 10-mg-vonoprazan maintenance period, the symptomatic non-relapse rates for acid reflux-associated and dysmotility symptom FSSG scores were 86.5 and 80.8%, respectively [6]. During the 48-week 10-mg-vonoprazan maintenance therapy, the symptomatic non-relapse rates for acid reflux-related symptom score of FSSG and acid reflux score of the Gastrointestinal Symptom Rating Scale at 48 weeks were 70.0 and 72.0%, respectively [15]. In another study, no significant difference was noted in the FSSG score between 8 and 52 weeks of 10-mg VPZ administration [16].
LDA- or NSAID-induced peptic ulcers
Vonoprazan (10 and 20 mg) was also as effective as lansoprazole (15 mg) in preventing LDA-induced peptic ulcer recurrence in a 24-week and long-term extension therapy study [19]. In the same way, non-inferiority of vonoprazan (10 and 20 mg) to prevent NSAID-related peptic ulcer recurrence was verified in patients receiving long-term NSAIDs in a 24-week study. Beyond that, it was effective and well-tolerated for longer than 1 year, with a safety profile similar to lansoprazole (15 mg) [20]. Both studies recommend a daily vonoprazan dose of 10 mg as the clinical dose.
Discussion
The results of this systematic review demonstrate non-inferiority of 8-week or 8-week treatment with vonoprazan versus PPIs in reflux oesophagitis or erosive oesophagitis healing. One study demonstrated higher vonoprazan (20 mg) effectiveness than lansoprazole (30 mg) treatment for severe erosive oesophagitis. In addition, 10-mg-vonoprazan therapy showed effectiveness in maintaining healed reflux oesophagitis for up to 52 weeks. Also, complete sustained heartburn relief was achieved sooner with vonoprazan than with lansoprazole during the first week of therapy. Therefore, our analysis may provide helpful information for clinicians, enabling them to offer other treatment choices to patients with GERD.
A systematic review with Bayesian network meta-analysis to estimate the comparative efficacy of treatments between PPI and vonoprazan was previously conducted by Miyazaki et al. [21]. This analysis showed that the GERD healing effect of vonoprazan is non-inferior to other PPIs, except for rabeprazole [21], but vonoprazan showed more effectiveness than most PPIs in the severe erosive oesophagitis patient group [21]. Another systematic review and meta-analysis made a direct comparison of the therapeutic effects and adverse events between vonoprazan 20 mg and PPIs. The researchers found that vonoprazan is non-inferior to PPIs as therapy for patients with GERD. Subgroup analysis for patients with severe oesophagitis at baseline showed significantly higher results for vonoprazan than for lansoprazole, with an RR of 1.14 (1.06–1.22). The safety outcomes for vonoprazan were similar to those for PPIs [22].
Gastroesophageal reflux disease adversely affects the quality of life of patients. Therefore, treatment with acid secretion suppressors can improve the quality of life related to gastrointestinal symptoms other than those involving the oesophagus [1, 4].
Furthermore, daily vonoprazan 10 mg can be considered the recommended clinical dose for preventing LDA- or NSAID-related peptic ulcer recurrence in at-risk patients. Vonoprazan has the potential to be a clinically useful alternative to PPIs, especially for patients with high-risk factors.
A major limitation of this study is the exclusion of all publications in languages other than English, Portuguese, or Spanish. In addition, conducting a meta-analysis would provide more robust results to determine the outcome of interest.
Conclusions
Our findings suggest that vonoprazan was effective and non-inferior to PPIs in healing reflux oesophagitis. It also can be considered for preventing LDA- or NSAID-related peptic ulcer recurrence in at-risk patients. Complete sustained heartburn relief was achieved sooner with vonoprazan than with PPIs during the first week of therapy. Also, vonoprazan showed better results than lansoprazole analysis for patients with severe oesophagitis.