Introduction
Acid-related diseases result from distinct but overlapping pathogenic mechanisms that involve acid effects on an oesophagogastric duodenal mucosa with diminished defence. While gastroesophageal reflux disease (GERD) represents the most frequently observed acid-related disorder, peptic ulcers and ulcers caused by the use of non-steroidal anti-inflammatory drugs (NSAIDs) and acetylsalicylic acid (ASA) are also important acid-related diseases [1, 2].
The symptoms associated with acid-related diseases affect health-related quality of life and work productivity. Serious complications such as oesophageal stricture, ulceration, Barrett’s oesophagus, or cancer may develop without effective treatment [3].
Proton pump inhibitors (PPIs) were introduced in the late 1980s and dramatically improved gastric acid-related conditions [4]. Nowadays, PPIs dominate in acid-related disease management worldwide [5–8]. However, despite their efficiency, PPIs may exhibit delay in symptom improvement, low bioavailability, fast metabolism, drug interactions, variable sustainability of acid suppression, enteric-coated pharmaceutical form, and more effective action on the nocturnal acidity breakthrough, among other limitations that together lead to unmet acid-related disease management needs [9]. In this scenario, potassium-competitive acid blockers (P-CABs) have emerged to promote a better antisecretory effect that addresses these unmet needs, including the rapid improvement of symptoms, refractory GERD, and nocturnal acid reflux related to disease management [10].
The first P-CAB used in clinical practice was revaprazan, and it has been available in South Korea and India since 2007, but there are no reports that it is more effective than PPIs for acid-related conditions [4]. Vonoprazan became available in Japan in 2015 to treat gastric ulcer, duodenal ulcer, and erosive oesophagitis, and to prevent low dose aspirin- or NSAID-induced peptic ulcer recurrence, and tegoprazan was approved as a treatment for GERD in South Korea in July 2018 [4, 11, 12].
Aim
Because of the advent of new P-CABs and their efficacy profile, we conducted a systematic review to assess the safety of vonoprazan in the management of patients diagnosed with GERD oesophagitis, with peptic ulcers, or those with ulcers induced by chronic use of aspirin or NSAIDs.
Material and methods
Study design
In order to answer to the proposed objectives, a systematic review of the available literature was conducted. The research question was defined according to the PICO model (population; intervention; control; outcome) [13]. Studies that evaluated vonoprazan safety when used as a treatment strategy for patients diagnosed with GERD oesophagitis were searched, along with studies that concerned vonoprazan treatment of peptic ulcers induced by chronic use of aspirin or NSAIDs or gastric/duodenal ulcer.
Search strategy
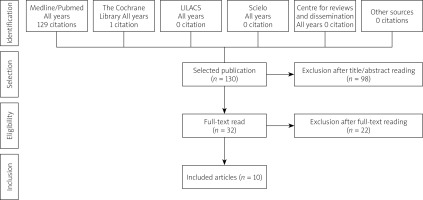
A literature search was conducted on 20 June 2021 using Medline via PubMed, Cochrane library, Lilacs, SciELO, and Centre for Reviews and Dissemination (CRD) electronic databases for the terms listed in Table I. Additionally, manual searches of bibliographic references and abstracts of selected publications complemented the electronic searches.
Table I
Search queries
Eligibility and inclusion and exclusion criteria
Studies to be considered eligible should meet the following inclusion criteria: meta-analyses, systematic reviews and phase III or IV randomized controlled trials (RCTs), or observational studies; studies involving patients using vonoprazan to treat GERD oesophagitis, gastric/duodenal ulcers or those in chronic use of aspirin or NSAIDs; and analysis with potassium pump inhibitor as a comparator or without comparator and safety endpoints. Furthermore, all articles under at least one of the following conditions were excluded: narrative reviews, guidelines, consensus articles, editorials, case reports, or case series; studies involving patients with Helicobacter pylori or with ulcers due to endoscopic submucosal dissection; studies using animal models; and articles published in other languages than English, Portuguese, and Spanish.
Results
Study selection
A total of 130 studies were retrieved from the database, including duplicates. After applying eligibility criteria, 32 articles were selected for full-text reading by 2 reviewers. Finally, 10 studies were included in this review (Figure 1).
Characteristics of studies
Table II shows the included studies list and the description of their general characteristics according to the comparator type. Of the 10 articles included in the analysis, 7 were compared to lansoprazole, 2 against different doses of vonoprazan, and 1 in a single arm. The number of patients ranged from 19 to 850, totalling 3618 patients included in the selected studies. Patients were followed for a period of up to 52 weeks.
Table II
Characteristics of selected studies and clinical profile of patients
| Author, year | Local | Population | Intervention | Comparator | Study design | Follow-up |
|---|---|---|---|---|---|---|
| Okanobu, 2020 [16] | Japan | 78 patients with endoscopically confirmed EE | Vonoprazan 20 mg (initial treatment) Vonoprazan 10 mg (maintenance treatment) | Vonoprazan 10 mg | RCT | 12 weeks |
| Xiao, 2020 [17] | Asia | 468 patients with endoscopically confirmed EE | Vonoprazan 20 mg | Lansoprazole 30 mg | RCT | 4 weeks |
| Mizuno, 2019 [22] | Japan | 50 patients aged ≥ 20 years, with RE refractory to PPIs who had no endoscopic evidence of erosive esophagitis after the administration of VPZ 20 mg od/4 weeks | Vonoprazan 20 mg (initial treatment) Vonoprazan 10 mg (maintenance treatment) | – | Open-label, prospective | 48 weeks |
| Ashida, 2018 [23] | Japan | 607 patients ≥ 20 years, who presented with endoscopically confirmed healed EE after vonoprazan 20 mg od/up to 8 weeks | Vonoprazan 10 mg Vonoprazan 20 mg | Lansoprazole 15 mg | RCT | 24 weeks |
| Kawai, 2018 [24] | Japan | 621 patients (439 in extension) with long-term LDA-associated peptic ulcers | Vonoprazan 10 mg Vonoprazan 20 mg | Lansoprazole 15 mg | RCT | 24 weeks |
| Mizokami, 2018 [25] | Japan | 642 patients receiving long-term NSAID therapy, who are at risk of peptic ulcer recurrence | Vonoprazan 10 mg Vonoprazan 20 mg | Lansoprazole 15 mg | RCT | 24 weeks |
| Soiza, 2017 [18] | Japan | 19 patients with PPI-resistant EE | Vonoprazan 20 mg | Vonoprazan 40 mg | RCT | 8 weeks |
| Miwa, 2017 [19] | Japan | 482 patients with gastric ulcer and 368 patients with duodenal ulcer | Vonoprazan 20 mg | Lansoprazole 30 mg | RCT | 8 weeks (gastric ulcer cohort) 6 weeks (duodenal ulcer cohort) |
| Ashida, 2016 [20] | Japan | 401 patients (305 in extension) with endoscopically confirmed EE | Vonoprazan 10 mg Vonoprazan 20 mg | Lansoprazole 30 mg | RCT | 52 weeks |
| Ashida, 2015 [21] | Japan | 732 patients ≥ 20 years with endoscopically confirmed EE | Vonoprazan 5 mg Vonoprazan 10 mg Vonoprazan 20 mg Vonoprazan 40 mg | Lansoprazole 30 mg | RCT | 8 weeks |
Vonoprazan use as initial therapy
Six studies considering the use of vonoprazan as initial therapy were included in the analysis [16–21]. Information about treatment-emergent adverse events is shown in Table III. Several adverse events with frequency > 5% reported in the studies are described in Table IV. Okanobu et al. reported the absence of adverse events during the study period but did not provide details on assessments, so it was not described in respective tables [16].
Table III
Overview of treatment-emergent adverse events and serious adverse events in studies using vonoprazan as initial therapy
| Author, year | Treatment | TEAEs | Leading to discontinuation | Liver function abnormalities | SAEs | Deaths | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (%) | Related (%) | Not related (%) | Mild (%) | Moderate (%) | Severe (%) | Total (%) | Related (%) | Not related (%) | (%) | Total (%) | Related (%) | Not related (%) | Leading to discontinuation (%) | (%) | ||
| Xiao, 2020 [17] | Vonoprazan 20 mg | 38.1 | 14.8 | 23.4 | 31.1 | 5.7 | 1.2 | 2.0 | 1.2 | 0.8 | 0 | 1.2 | 0 | 1.2 | 0.8 | 0 |
| Lansoprazole 30 mg | 36.6 | 11.5 | 25.1 | 30.2 | 5.5 | 0.9 | 1.7 | 0.9 | 0.9 | 0.9 | 1.3 | 0 | 1.3 | 0.4 | 0 | |
| Iwakiri, 2017 [18] | Vonoprazan 20 mg | 44.4 | 11.1 | 33.3 | 44.4 | 0 | 0 | 0 | – | – | – | 0 | – | – | – | – |
| Vonoprazan 10 mg | 60.0 | 10.0 | 50.0 | 30.0 | 30.0 | 0 | 10.0 | – | – | – | 0 | – | – | – | – | |
| Miwa, 2017 [19] | Vonoprazan (GU) | 26.6 | 6.6 | – | 21.7 | 3.7 | 1.2 | 2.0 | – | – | – | 2.5 | 0.0 | – | 0.8 | 0.0 |
| Lansoprazole (GU) | 33.2 | 5.9 | – | 30.7 | 2.1 | 0.4 | 0.8 | – | – | – | 1.7 | 0.8 | – | 0.8 | 0.0 | |
| Vonoprazan (DU) | 34.4 | 9.3 | – | 27.9 | 4.4 | 2.2 | 2.7 | – | – | – | 3.3 | 0.5 | – | 1.6 | 0.5 | |
| Lansoprazole (DU) | 28.6 | 4.9 | – | 23.8 | 4.9 | 0 | 1.1 | – | – | – | 2.2 | 0.0 | – | 0.5 | 0.0 | |
| Ashida, 2016 [20] | Vonoprazan | 22.2 | 6.8 | – | – | – | – | 1.0 | – | – | – | 0.0 | – | – | – | 0.0 |
| Lansoprazole | 22.3 | 5.9 | – | – | – | – | 1.5 | – | – | – | 1.5 | – | – | – | 0.0 | |
| Ashida, 2015 [21] | Vonoprazan 5 mg | 39.9 | 6.1 | – | – | – | 0.7 | – | – | – | 0.7 | – | – | – | – | |
| Vonoprazan 10 mg | 42.8 | 9.0 | – | – | – | – | 3.4 | – | – | – | 0.0 | – | – | – | – | |
| Vonoprazan 20 mg | 47.4 | 10.4 | – | – | – | – | 7.1 | – | – | – | 1.9 | – | – | – | – | |
| Vonoprazan 40 mg | 37.9 | 4.8 | – | – | – | – | 1.4 | – | – | – | 1.4 | – | – | – | – | |
| Lansoprazole 30 mg | 43.9 | 5.8 | – | – | – | – | 2.9 | – | – | – | 0.7 | – | – | – | ||
Table IV
Adverse events with frequency > 5% reported in at least 1 study, considering those using vonoprazan as initial therapy
| Variable | Xiao, 2020 [17] | Iwakiri, 2017 [18] | Ashida, 2016 [20] | Ashida, 2015 [21] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VPZ (%) | LPZ (%) | VPZ 20 mg (%) | VPZ 40 mg (%) | VPZ (%) | LPZ (%) | VPZ 5 mg (%) | VPZ 10 mg (%) | VPZ 20 mg (%) | VPZ 40 mg (%) | LPZ (%) | |
| GI disorders | 18.4 | 19.1 | – | – | – | – | – | – | – | – | – |
| Blood gastrin increased | 5.3 | 1.7 | – | – | – | – | – | – | – | – | – |
| Gastric mucosal lesions | – | – | 0.0 | 10.0 | – | – | – | – | – | – | – |
| Gastritis | – | – | 11.1 | 0.0 | – | – | – | – | – | – | – |
| Oedema, peripheral | – | – | 11.1 | 0.0 | – | – | – | – | – | – | – |
| Pyrexia | – | – | 0.0 | 10.0 | – | – | – | – | – | – | – |
| Food allergy | – | – | 0.0 | 10.0 | – | – | – | – | – | – | – |
| Fall | – | – | 0.0 | 10.0 | – | – | – | – | – | – | – |
| Ligament sprain | – | – | 0.0 | 10.0 | – | – | – | – | – | – | – |
| Flank pain | – | – | 0.0 | 10.0 | – | – | – | – | – | – | – |
| Muscular weakness | – | – | 0.0 | 10.0 | – | – | – | – | – | – | – |
| Skin papilloma | – | – | 0.0 | 10.0 | – | – | – | – | – | – | – |
| Epistaxis | – | – | 11.1 | 0.0 | – | – | – | – | – | – | – |
| Rhinalgia | – | – | 11.1 | 0.0 | – | – | – | – | – | – | – |
| Psoriasis | – | – | 11.1 | 0.0 | – | – | – | – | – | – | – |
| Infections and infestations | – | – | – | – | 6.3 | 7.4 | – | – | – | – | – |
| Nasopharyngitis | – | – | – | – | – | – | 8.1 | 10.3 | 9.7 | 8.3 | 10.1 |
Similar frequencies of treatment-emergent adverse events, serious adverse events, and those leading to treatment discontinuation were reported by the studies (Table III). Gastrointestinal disorders were the most frequently observed events (Table IV).
Vonoprazan use as maintenance therapy
Considering vonoprazan as maintenance therapy, a total of 4 studies were included in the analysis [22–25]. Table V shows information about treatment-emergent adverse events, while several adverse events with frequency > 5% reported in the studies are described in Table VI. As observed for vonoprazan use as initial therapy, a similar pattern of adverse events occurrence was found in several comparator arms. Nasopharyngitis was the most frequently observed event (Table VI).
Table V
Overview of treatment-emergent adverse events and serious adverse events in studies using vonoprazan as maintenance therapy
| Author, year | Treatment | TEAEs | Leading to discontinuation Total (%) | Liver function abnormalities (%) | SAEs | Deaths (%) | ||
|---|---|---|---|---|---|---|---|---|
| Total (%) | Related (%) | Total (%) | Related (%) | |||||
| Mizuno, 2019 [22] | Vonoprazan 10 mg | 6.0 | 6.0 | – | – | 0 | – | 0 |
| Ashida, 2018 [23] | Vonoprazan 10 mg | 54.0 | 10.4 | 2.5 | – | 2.5 | 0 | |
| Vonoprazan 20 mg | 58.8 | 10.3 | 3.9 | – | 2.0 | 0 | ||
| Lansoprazole 30 mg | 51.2 | 11.4 | 4.0 | – | 2.0 | 0 | ||
| Kawai, 2018 [24] | Vonoprazan 10 mg | 87.6 | 16.3 | 7.9 | – | 16.3 | 2.0 | 0.5 |
| Vonoprazan 20 mg | 87.1 | 19.3 | 7.4 | – | 15.8 | 2.0 | 0 | |
| Lansoprazole 30 mg | 84.8 | 24.4 | 9.2 | – | 14.7 | 1.4 | 0 | |
| Mizokami, 2018 [25] | Vonoprazan 10 mg | 84.4 | 17.4 | 4.1 | – | 8.3 | 0.9 | – |
| Vonoprazan 20 mg | 82.5 | 17.5 | 12.7 | – | 14.2 | 0.9 | – | |
| Lansoprazole 30 mg | 88.1 | 19.0 | 7.6 | – | 8.6 | 0 | – | |
Table VI
Adverse events with frequency > 5% reported in at least 1 study, considering those using vonoprazan as maintenance therapy
| Variable | Ashida, 2018 [23] | Kawai, 2018 [24] | Mizokami, 2018 [25] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Vonoprazan 10 mg (%) | Vonoprazan 20 mg (%) | Lansoprazole 30 mg (%) | Vonoprazan 10 mg (%) | Vonoprazan 20 mg (%) | Lansoprazole 30 mg (%) | Vonoprazan 10 mg (%) | Vonoprazan 20 mg (%) | Lansoprazole 30 mg (%) | |
| Nasopharyngitis | 16.8 | 13.2 | 13.9 | 29.7 | 31.2 | 31.3 | 32.1 | 27.8 | 29.0 |
| Diarrhoea | 3.0 | 2.5 | 5.5 | 7.4 | 9.4 | 12.0 | 5.0 | 7.1 | 6.7 |
| Constipation | 1.0 | 1.5 | 2.0 | 6.4 | 8.4 | 7.4 | 6.9 | 3.3 | 2.4 |
| Upper respiratory tract inflammation | 4.0 | 2.0 | 1.5 | 5.9 | 6.4 | 4.6 | 5.5 | 6.6 | 3.3 |
| Fall | 4.0 | 1.0 | 0.5 | 5.4 | 4.0 | 6.0 | 10.1 | 8.5 | 8.6 |
| Back pain | 1.5 | 2.5 | 0.5 | 4.0 | 7.9 | 2.3 | 3.2 | 6.1 | 2.9 |
| Elevated creatine phosphokinase | 2.0 | 2.9 | 1.0 | 4.0 | 5.4 | 4.6 | 4.1 | 3.3 | 5.2 |
| Contusion | 2.5 | 1.0 | 1.5 | 3.5 | 3.5 | 6.5 | 7.8 | 6.6 | 9.5 |
| Seasonal allergy | 2.0 | 1.0 | 1.0 | - | - | - | 3.7 | 7.1 | 3.8 |
| Contact dermatitis | – | – | – | – | – | – | 3.7 | 5.7 | 2.9 |
Discussion
This systematic review was conducted to assess the safety of vonoprazan in the management of patients diagnosed with GERD oesophagitis, gastric/peptic ulcers, or peptic ulcers induced by chronic use of aspirin or NSAIDs. Considering the drug efficacy profile and safety data available in the current literature, this study adds important knowledge to disease management.
P-CABs were first described in the 1980s with SCH28080. This compound showed the capacity to inhibit gastric acid secretion in humans and animals, beyond inhibition of H+,K+-ATPase via competitive interaction with K+ site of the enzyme. Despite the favourable results, hepatic toxicity after prolonged administration led to the discontinuation of clinical studies. More recently, several SCH28080 derivative compounds were developed including vonoprazan, which is one of the drugs from P-CABs class available for use in clinical practice [12, 26].
Considering the initial concern about hepatic toxicity, we found 1 study in our systematic review that assessed the occurrence of this outcome. Xiao et al. reported safety events among patients with the diagnosis of erosive oesophagitis treated with P-CABs and PPIs. Liver function abnormalities were observed in 0.9% of the 235 patients treated with lansoprazole 30 mg, but no events were observed in the group treated with vonoprazan 20 mg (n = 244) [17].
The frequency of treatment-emergent adverse events in general ranged from 6.0% to 87.6%, and those related to the treatment from 4.8% to 19.3%. The great variability observed across the studies’ estimates is related to whether the treatment was an initial or a maintenance therapy. Regarding serious adverse events, the occurrence ranged from 0.0% to 16.3% and from 0.0% to 2.0% for total and those related to treatment, respectively [16–25].
In general, the safety profile of vonoprazan was similar to that observed for lansoprazole, a potent PPI [17, 19–21, 23–25]. This finding confirms previous data reported in the current literature. He et al. conducted a systematic review and meta-analysis to compare the efficacy, safety, and tolerance of vonoprazan with PPIs in the treatment of peptic ulcers resulting from endoscopic submucosal dissection. The study reported a similarity between groups, with an insignificant relative risk for adverse events (RR = 0.65; 95% CI: 0.31–1.38) [3]. Recently, Chen et al. also reported that vonoprazan and PPIs have similar safety profiles (RR = 1.08; 95% CI: 0.96–1.22) in a meta-analysis considering patients with GERD [27].
All the studies included in this review were conducted in eastern countries. This may be pointed out as a matter of concern because populations from such countries have slower metabolizers and the safety profile may differ from that in western populations [28]. Jenkins et al. conducted 2 phase I studies in order to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of TAK-438 in healthy Japanese and non-Japanese men. Treatment-emergent adverse events were observed among 9 of 60 subjects in the Japanese cohort and in 10 of 48 subjects in the UK cohort, while no serious adverse events were observed in both studies [29]. These data suggest that the metabolization profile of eastern populations has no impact on the safety of vonoprazan.
Despite the important results observed in the present study, some limitations should be highlighted. Language limits led us to exclude all publications in languages other than English, Portuguese, or Spanish. This may be considered as a major limitation of this study. In addition, a meta-analysis of the available data would provide more robust results to determine the outcome of interest.
Conclusions
The study findings suggest that vonoprazan has a favourable safety profile, especially when compared to PPIs (such as lansoprazole). Thus, considering efficacy data previously reported, it may be considered as a good and safe therapeutic option for the management of acid-related diseases.