Dear editor,
C-peptide has become an increasingly important parameter in diabetes-related clinical trials involving interventions and very low levels (< 10 pmol/l) have been associated with fewer incidences of hypoglycaemia and diabetes complications like retinopathy and nephropathy [1]. To measure C-peptide, a wide range of assays with varying sensitivities is currently available. When searching the literature for an appropriate C-peptide assay for one of our clinical studies we noticed that many publications do not provide technical details of the used C-peptide assay. Indeed, many studies do not even mention the specific assay used at all. This prompted us to evaluate which C-peptide assays are currently available and to what extent information is provided about these assays used in publications in the diabetes research field.
We searched PubMed with the search terms “assay”, “C-peptide” and “diabetes” between 01-01-2016 and 31-12-2020, only including publications in the English language. Abstracts of the eligible publications were screened. Exclusion criteria were: comparisons between assays, case studies, neonatal and umbilical blood, reviews, meta-analyses, case reports, animal and cell models, protocols, non-diabetes research, healthy individuals, absence of abstract, C-peptide in urine, new method developments, health economics/database studies and mass spectrometry method development. The term “C-peptide” had to be present in the abstract. The full texts of the remaining publications were searched for the presence of information on the used C-peptide assay. Where available, technical details (measurement range, detection limit, precision) and lowest and highest C-peptide concentrations were collected from text, tables and graphs.
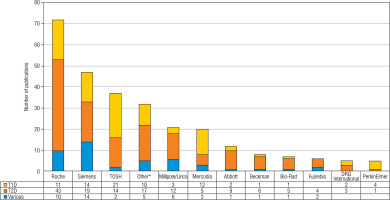
Of 1,289 eligible publications, 515 were included (raw results can be provided upon request). Of these, 167 (32%), 277 (54%) and 71 (14%) reported C-peptide measurements in the context of respectively T1D, T2D and other forms of diabetes (e.g. Latent Autoimmune Diabetes in Adults [LADA], Maturity Onset Diabetes of the Young [MODY], gestational diabetes). Overall, the used assay was not mentioned in 240 (47%) publications (Fig. 1). About half of the T1D and T2D publications and one third of the publications on other forms of diabetes did not specify the assay. Very few of the publications that did mention the used assay provided full technical specifications of assay performance. Information on the detection limit, analytical range and precision (mostly intra- and inter-assay variability) were mentioned in 61 (12%), 9 (2%) and 57 (11%) of publications, respectively. In T1D publications the detection limit was mentioned more frequently and C-peptide concentrations less frequently, compared with publications on T2D/other types of diabetes. There were no clear differences for measurement range and precision. Measured C-peptide concentrations were not mentioned in 115 publications (22%). Most C-peptide measurements in the diabetes research field are performed with chemiluminescence immunoassays from Roche, Siemens and Tosoh (Fig. 2). Mercodia is the most frequently mentioned manufacturer in the T1D field. After chemiluminescent assays, ELISA is the most commonly used methodology.
Figure 1
Numbers and percentages of publications by mention of assay, technical specifications (total n = 515) and C-peptide concentrations (total n = 508; 8 publications had issues with the used unit and were not included in the graph)
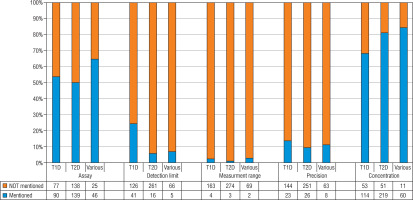
Figure 2
Suppliers of C-peptide assays mentioned in the included publications
* Suppliers mentioned in < 5 publications were included in the category ‘Other’ (Abnova, Biosource/Thermofisher, Biosystems, Diagnostic Systems Laboratories, DIAsource, DPC, IBL, Invitron, Linco, Nanjing Jian Cheng Institute, Snibe, Technogenetics, Beijing North Institute of BiologicalTechnology, Bio-Ekon Inc., Chemux, Cisbio International, Diasorin, Kyowa Medix, Mesoscale, Monobind, Tecan/IBL, Alpco)
T1D – type 1 diabetes; T2D – type 2 diabetes; various – other type of diabetes
A possible explanation for the lack of technical details may be that in many cases, e.g. in the context of T2D, measured C-peptide levels will be well above the detection limits of the well-established commercial assays run on random access analysers in routine laboratories [2], although we did not find that publications reporting higher C-peptide values specified the used assay less frequently. In >20% of publications measured C-peptide concentrations were not reported, often because C-peptide concentrations thresholds were used and results were reported as percentages of people below and above the threshold, without providing the measured values. A common problem in laboratory medicine is the use of absolute threshold values for analytes in guidelines without specifying the assay that was used to obtain these thresholds. Many laboratory tests, including C-peptide assays are poorly standardized and can have large variability between assays. The choice of assay can have a large impact on clinical decision making when a single threshold is used regardless of which assay is chosen. A person with diabetes can have C-peptide levels below the threshold in one assay and above the threshold using another. Disregard for the inherent variability of laboratory tests from different manufacturers can potentially lead to misdiagnosis, suboptimal patient treatment and even iatrogenic harm. Also, in many publications the area under the curves (AUCs) of C-peptide concentration time courses were calculated, or certain indices (e.g. C-peptide reactivity index [CPR], homeostasis model assessment of C-peptide secretion [HOMA-CR]). We noted that frequently the methods for measuring other analytes were specified, but not for C-peptide.
Already in 2008, when there was a less urgent need for measuring very low levels of C-peptide, Little et al. found that C-peptide measurements acquired by various methods and laboratories do not always agree [3] and they advocated a more generalized standardization program. This should include the WHO International Standard for human C-peptide (batch 13/146) [4]. We recently compared 2 commercially available assays and found that one of the assays did not meet the detection limit (limit of quantitation [LOQ]) claimed by the manufacturer [5], although both assays correctly measured the WHO standard for C-peptide.
Summarizing we conclude that many C-peptide measurements in diabetes research are presented without mention of the specific assay used and/or its analytical characteristics. Due to poor standardization of C-peptide assays it is therefore difficult to compare different studies or reproduce their results. In light of increasing need for C-peptide assays capable of measuring very low levels, we stress the importance of recognizing the variability between different C-peptide assays and taking these differences into account when formulating thresholds, interpreting patient data and presenting new research results. Including assay information in research papers will benefit the correct interpretation of results. Finally, we propose further standardization and harmonization of C-peptide assays to obtain better concordance across different methodologies.

 ENGLISH
ENGLISH






