Introduction
The obesity epidemic in paediatrics has caused great concern, and its prevalence has increased alarmingly, affecting developed and developing countries, secondary to lifestyle changes, reduced activities, and sedentary lifestyles [1]. In paediatrics, the ratio between weight and height defines obesity, estimated as ≥ 95 by body mass index (BMI) for sex and age. Overweight is defined as BMI ≥ 85 according to CDC (Centres for Disease Control and Prevention) growth tables. BMI is obtained by dividing the weight [kg] by the height [m] squared. In Mexico there are no specific parameters, and CDC values are used as a reference [2].
The growing prevalence and severity of obesity results in an increase in chronic degenerative diseases, particularly of the metabolic and cardiovascular systems, and especially sleep-related breathing disorders (SDB) [3].
The evaluation of SDBs requires a full clinical history and completion of sleep diaries by caregivers and ancillary diagnostic tests such as nocturnal polysomnography (which is considered as a gold standard test), nocturnal electroencephalography with recording, oximetry, and sleep latency tests [4]. The prevalence of SDB is unknown in Mexico, but the prevalence of its main symptom, snoring, is variable, at between 7% and 16.7% in children aged 6 months to 13 years and between 5% and 14.8% in teenagers [5].
Obstructive sleep apnoea (OSA) is considered a SDB, ranging from partial to complete upper airway obstruction; OSA is defined by the absence of nasal airflow, despite chest and abdominal wall movements, for at least 2 respiration periods. Obstructive hypopnoea represents a 50% decrease in nasal airflow with respect to the initial value, accompanied by a 3% decrease in oxygen saturation and/or hypoexcitation [6]. The frequency of apnoea and hypopnoea episodes per hour of sleep is expressed by the apnoea/hypopnoea index (AHI) on polysomnography [6].
The lack of access to polysomnography has led to the development of clinically useful scales, such as the Paediatric Sleep Questionnaire (PSQ), as a support for identifying and diagnosing SDB in the absence of polysomnography [7]. It has 2 versions: a reduced form of 22 questions, which is preferred, and an extended form that addresses several conditions such as excessive daytime sleepiness, behavioural disorders, parasomnias, insomnia, etc. [7].
Despite the prevalence of obesity in Mexican paediatrics and the wide access to the PSQ, there are no studies on the prevalence of SDB, and its impact and specific risk factors for the Mexican population are unknown.
Aim of the study
We sought to determine the association between obesity and SDB in paediatric patients being followed up at the Obesity Clinic of the University Hospital “Dr. José Eleuterio González” of the Universidad Autónoma de Nuevo León (UANL), evaluating its prevalence according to the presence or absence of obesity and SDB.
Material and methods
This observational and analytical study, with retrospective and cross-sectional directionality, was performed by review of clinical records of patients aged 2–16 years with follow-up in the paediatric obesity clinic from March 2019 to 2021.
A tablet was used to apply the instrument to family members and/or caregivers of children with complete clinical history and > 2 visits in the year prior to the approach. Those who did not meet the characteristics and had obstructive respiratory morbidity (asthma, bronchial hyperreactivity, tonsillar hypertrophy), diabetes mellitus, and/or use of medications that favour weight gain were excluded.
Authorization was required from the UANL Ethics and Research Committee, registration #PE21-00045. Data were collected and entered into Microsoft (MS) Excel and identified by initials, and access was limited. Data confidentiality was assured.
Sample size
It was calculated with an infinite population proportion formula. Expecting to find prevalence of SDB 17.6%, requiring bilateral significance of 0.05 and power of 80% given a delta of 0.1; finding a minimum sample of 56 subjects when considering validation of the PSQ [7].
Variables
Two groups were used; one with SDB and one without SBD, assigned by PSQ in a 22-item version, considering 8 affirmative answers as a cut-off point, and with sensitivity 86%, specificity 85%, and internal consistency of 0.88 with Cronbach’s α [7]. The PSQ questions are answered simply and concisely “yes”, “no”, or “don’t know” except for questions on inattention and hyperactivity, because they use criteria from the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV), corresponding to Likert-type answers (never, sometimes, often, and almost always). The PSQ is a reliable and validated instrument that requires between 20 and 30 minutes to complete.
Statistical analysis
SPSS version 25.0 was used from data in MS Excel. The normality of distribution was evaluated with the Kolmogorov-Smirnov test to decide on the use of parametric tests or their nonparametric equivalent. Sociodemographic and anthropometric variables were included as descriptive variables, and presented as frequencies and percentages when qualitative; for quantitative variables, the mean, standard deviation, median, and range were used. For qualitative variables, χ2 or Fisher’s exact test was used for independent groups. For quantitative variables, Student’s t-test was used for independent samples with parametric distribution and the Mann-Whitney U test for nonparametric distribution. After univariate analysis, a logistic regression adjusted for sex and age was performed to identify related variables as predictors or risk factors for SDB; p < 0.05 was considered statistically significant.
Results
Sixty-four patients were evaluated and included, and the presence of SDB was found in 27 (42.18%). The demographic characteristics can be seen in Table I.
Table I
Anthropometric variables
[i] Quantitative parametric variables were approached using Student’s t-test. Quantitative nonparametric variables were approached through the Mann-Whitney U test. Qualitative nonparametric variables were addressed through χ2 tests or Fisher’s Exact Test. An asterisk represents a significant p-value < 0.05.SD – standard deviation, IQR – interquartile range.
The variables that presented the most marked statistical difference were mean weight (54.18 vs. 41.51 kg, p = 0.018), BMI (24.34 vs. 20.52, p = 0.017), mean BMI percentile (97 vs. 77, p ≤ 0.0001), and height percentile (63.13 vs. 38.36, p = 0.002) for the group with obesity and SDB.
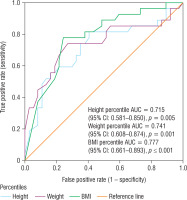
Using ROC (receiving operating characteristic) the following were found: BMI percentile (area under the curve [AUC] = 0.777, 95% CI: 0.661–0.893; p = 0.0001), weight percentile (AUC = 0.741, 95% CI: 0.608–0.874; p = 0.001), and height percentile (AUC = 0.715, 95% CI: 0.581–0.850; p = 0.005) as the best-behaved variables to classify SDB (Fig. 1).
Figure 1
Receiver operating characteristic (ROC) curve as predictors for the presence of sleep disorders
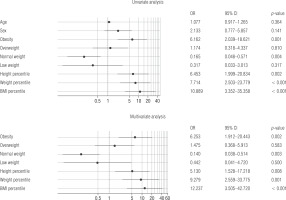
In multivariate logistic regression, obesity (odds ratio [OR] = 6.25, 95% CI: 1.91–20.44; p = 0.002), BMI ≥ 93.5 percentile (OR = 12.237, 95% CI: 3.5–42.72), percentile height ≥ 38 (OR = 5.13, 95% CI: 1.52–17.21; p = 0.001), and percentile weight ≥ 75 (OR = 9.29, 95% CI: 2.55–33.77; p < 0.001) were found to be associated risk factors (Fig. 2).
Discussion
The present study evaluates the presence of SDB in paediatric patients with obesity in Mexico. Obesity in paediatrics is known to be a public health problem. In school-age children, comprising 5–11 years, the prevalence of obesity was 18.6%, and for adolescents between 12–19 years it was 12.8% in 2016 [8], the same year in which the prevalence of obesity and overweight in children worldwide reached 340 million as a result of changes in eating habits, socioeconomic vulnerability, and sedentary lifestyle [8, 9]. Patients with overweight and normal weight under follow-up were also evaluated, revealing that the factors most associated with SDB were the presence of high BMI percentile and the ratio of weight and height percentiles. Males were found to have a slight predilection for presenting SDB, although this was not statistically significant, unlike adults in whom the predilection is markedly male, in whom SDB is identified as a factor for cardiovascular, pulmonary, and other chronic degenerative diseases such as stroke [10, 11].
The presence of obesity was the majority in the group with SDB; it represented 63% (17) of the total, while in the group without SDB, the normal weight group was the most represented, with 51.4% (17) of the total. The findings of the presence of obesity and SDB are consistent with those reported by Martínez-Cuevas et al. [11], who found a tendency among obese children to sleep less than non-obese children with SDB, decreased coexistence, less rest during sleep, and more marked metabolic alterations. This was related to the appearance of chronic degenerative diseases in adulthood or even in childhood/adolescence, with the impact of SDB being greater for children with obesity because it is related to disorders of lipid and carbohydrate metabolism, producing insulin resistance, similarly to that seen in adults with SDB and obesity, leading to cardiovascular consequences and a tendency to obesity in adulthood [12].
Childhood obesity is also related to low personality self-regulation, low reactivity, negative social perception from one’s own point of view, social isolation, anxiety, depression, and even eating disorders, which can be related to difficulty falling asleep or resting, especially if the child is bullied [13].
Detection and prevention are of fundamental importance; poor sleep quality is associated with deficits in verbal working memory, compromising learning and neurocognitive development. The presence of SDB appears to be of primary importance for preschool- and school-age children because it is associated with disorders such as attention deficit hyperactivity disorder (ADHD), behavioural disorders, and poor school performance [14, 15]. Children with SDB are reported to be tired most of the day even if they do not engage in physical activities, they are unwilling to play in a group, and have difficulty learning new skills. In addition, SDB is a risk factor for the development of dementia in old age [15–17]. It is relevant to highlight that 42% of the sample studied presented SDB – a higher prevalence than in other populations [17]. In paediatric patients with obesity, the prevalence of 63% is similar to that reported by others, such as Caminiti et al. [18] in Argentina, who found a prevalence of 55.2% in 58 children, with no significant differences in age or sex. Other studies show a lower prevalence of SDB – between 39.9% and 42%, while others report a prevalence of up to 80% [19, 20]. Regarding the risk of SDB in patients with obesity, we detected a 6.2-fold higher risk, compared to 1.2-fold found by other authors [21]. While the only similar study found in Mexico, and the only one to the authors’ knowledge, would mark a prevalence of 22% in children [22]. Population-based, experimental and interventional studies show evidence that shorter sleep duration increases the risk of obesity in children and adolescents [23]. Theories linking SDB and obesity aetiologically describe complex interactions between inflammatory pathways, oxidative stress, environmental factors, and genetic determinants [10].
It is remarkable that, despite the high prevalence of childhood obesity, there are no statistics that evaluate a common complication: SDB [8]. Our aim is to expose the need to identify SDB, to guide the use of tools such as PSQ mainly in children with obesity, and to establish the PSQ as a tool of epidemiological utility in the face of the high cost and difficult access to polysomnography [7, 8]. We suggest emphasizing screening and timely care of paediatric patients from the first level of care, to prevent the appearance of the multiple disorders described at an early age and their persistence in adulthood, when complications become more frequent. It is necessary to underline the presence of SDB as possible in childhood, and that its prevention, high suspicion, and weight loss are important and necessary action lines.
Limitations include a polarized population, small sample size, and difficulty to extrapolate results; prospective and multicentre studies are needed to assess the real impact of SDB in paediatric patients with obesity.
Conclusions
Paediatrics in our population show a marked risk of presenting SDB, secondary to a high prevalence of obesity and subsequent pathophysiology. BMI is a predictor of great importance that should be evaluated in primary care and by paediatricians in case of suspicion of SDB, making use of the application of the PQS in a clinical context for screening of complications in paediatric patients with obesity because it is a validated instrument that is easy to apply in the physician’s office.

 ENGLISH
ENGLISH