The hyperosmolar hyperglycaemic state (HHS) is a very severe condition, characterised by hyperosmolality, hyperglycaemia and dehydration without significant ketosis [1]. HHS is most often an acute complication of newly diagnosed or decompensated type 2 diabetes, but it can also develop in type 1 diabetes (T1DM). The population of children hospitalised due to HHS in the USA between the years 1997 to 2009 increased by 52.4% [2]. In certain conditions the development of HHS might be initiated by factors such as: infections (most commonly pneumonia [40–60%] or urinary tract infections [5–16%]), obesity, the metabolic syndrome, pancreatitis, dialysis, mental disorders, organic central nervous system changes, various medications (such as: corticosteroids, thiazide diuretics, atypical antipsychotics and sympathomimetic agents), total parenteral nutrition, and conditions associated with difficult fluid supplementation [2–5]. In addition, recent case reports suggest that some children with T1DM may exhibit symptoms of HHS if high-carbohydrate-containing beverages are ingested to satisfy thirst and replace urinary losses prior to diagnosis [2].
The first case of HHS in a child was described in 1966, and since then it has been diagnosed in a rising number of paediatric patients [3]. Currently, it is estimated to be the cause of hospitalisation in approximately 1% of patients with diabetes [2, 4, 6]. Late diagnosis and treatment lead to the high mortality of HHS: 50–60% [5].
HHS is a result of insulin deficiency, elevated levels of counter-regulatory hormones, increased gluconeogenesis and glycogenolysis, which lead to increasing hyperglycaemia [2, 4, 7]. Due to the elevated concentration of glucose and osmolality in the extracellular fluid, osmotic pressure increases and water ejects from the cells [2]. Prolonged osmotic diuresis due to glucosuria results in significant hypernatraemic dehydration and other electrolyte disorders [4]. Osmolality is a good indicator for monitoring the severity of the disease and the progress of the treatment [7].
A 14.5-year-old girl (spontaneous vaginal delivery, 41st week of gestation, mother’s 1st pregnancy, complicated by anaemia, maternal urinary tract infection and premature contractions, born small for the gestational age – birth weight of 2550 g, according to the World Health Organization’s Child Growth Standards, the infant weight corresponds to the 5th percentile and 1.6 SD below median weight for gestational age, Apgar score of 10 points), with a congenital cytomegalovirus infection, cerebral palsy (level five according to the Gross Motor Function Classification System (GMFCS) and epilepsy. Last hospitalisation at the age of 9 years due to a prolonged status epilepticus – the patient was discharged with a recommendation of continuous multi-specialist control.
Recently, the patient’s parents reported a medical emergency because she was apathetic and did not respond to stimuli – the symptoms increased gradually over the prior 2–3 days. The patient also refused to ingest any food, which led the parents to attempt to give her large amounts of high-carbohydrate beverages (750 ml of sweetened juice over a short period of time).
Laboratory tests from the emergency department of the regional hospital revealed mild metabolic acidosis, hypernatraemia, hyperglycaemia and prerenal kidney failure (Table I). The patient received a bolus of 0.9% NaCl (20 mL/kg), followed by an infusion composed of 0.45% NaCl, 5% glucose and short-acting insulin, and was transferred to the Department of Children’s Diabetology and Pediatrics with a diagnosis of diabetic ketoacidosis (DKA) in the course of new-onset diabetes.
Table I
Laboratory test in the emergency department of the regional hospital and the emergency department of our hospital
* 0.10.5 ng/ml – possibility of infection requiring antibiotic therapy ** osmolality calculated using a formula [8]
Control laboratory tests were performed at the emergency department of our hospital (Table I). Following an anaesthetic consultation, the patient received an infusion of water for injection. On admission to the Department of Children’s Diabetology and Pediatrics the patient was unconscious and in very severe condition. Physical examination revealed extreme cachexia (body weight of 13.8 kg, corresponding to < 3rd percentile and –2.18 standard deviation (growth chart for cerebral palsy GMFCS V, fed orally)) [9], signs of negligence, four-limb spastic paralysis, muscular atrophy and contracture, xerostomia, extensive oral candidiasis and vulvar mycosis, Tanner stage: M1 and P2.
Laboratory tests performed at the Department of Children’s Diabetology revealed mild ketonemia (0.2 mmol/l), hyperglycaemia (424 mg/gl), high white blood cell count (21.3 × 103/µl), mild anaemia, hypernatraemia (168 mmol/l), hypermagnesaemia (1.34 mmol/l), hyperchloraemia (136 mmol/l), hypophosphataemia (0.49 mmol/l) and normal concentrations of albumin and total protein. Full diabetes diagnostic was postponed due to the severe and life-threatening condition of the patient.
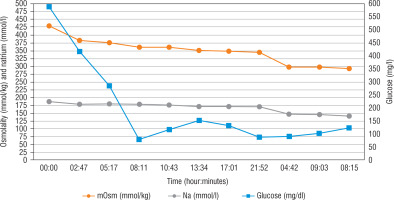
HHS was diagnosed and appropriate treatment was implemented as recommended by the 2018 Consensus Guidelines of the International Society for Pediatric and Adolescent Diabetes (ISPAD) [1]. Fluid deficits should be replenished faster in HHS than in DKA, therefore the fluid deficits were initially supplemented with isotonic solutions, combined with the monitoring of the water and electrolyte balance, osmolality and glucose levels.
Optimally, the rate of decreasing serum sodium concentrations in hypernatraemic dehydration should equal 0.5 mmol/h (Fig. 1). Glucose and insulin flows (short-acting insulin 0.025–0.05 U/kg/h) were modified based on the results of the laboratory tests (performed every 2–3 hours) to obtain a reduction of glucose levels at a rate of 75–100 mg/dl/h. Considering the risk of venous thrombosis, low molecular weight heparin was administered in a prophylactic dose. Although the elevated WBC might have been a result of dehydration and blood concentration, empirical antibiotic therapy and antifungal therapy were initiated as well. Potassium supplementation was administered, as well as calcium, phosphorus and magnesium supplementation in the following days. After 6 hours of intensive rehydration, the patient received only very low doses of insulin, but eventually did not require insulin at all. Following the gradual correction of electrolyte imbalance, natraemia dropped to 152 mmol/l in venous blood after a day, and to 136 mmol/l on the 3rd day. Normalisation of other laboratory test results and improvement of the patient’s general condition were observed as well.
The patient was transferred to the Pediatric Gastroenterology Department at the same hospital due to her life-threatening cachexia. Since stabilising the general and nutritional status of the patient was of paramount importance, the extended diabetological diagnostics was postponed and the parents were ordered to take the child to the hospital’s diabetological outpatient clinic after discharge from hospital. Due to the high risk of refeeding syndrome development, the caloric content of the patient’s meals was increased very slowly. Normal glucose levels were observed during the entire hospitalisation period and the patient did not require insulin. After the patient’s state was stabilised, percutaneous endoscopic gastrostomy (PEG) was performed under general anaesthesia without complications. The patient was discharged from hospital after nearly a month. During this time, she gained two kilograms (15 kg, –2.16 standard deviation according to the growth chart for cerebral palsy GMFCS V, tube-fed) [9]. The following recommendations were issued on discharge: constant care in the ambulatory nutrition centre and scheduled control visits in the hospital’s gastroenterology and diabetological outpatient clinics.
Unfortunately, most likely due to her chronic disorders, the patient suffered sudden death at home two months later. Parents did not wish to carry out an autopsy or any other examination. According to the information provided by the caregivers approximately three weeks before death, the patient was in very good condition and was gradually gaining weight. Considering the severe cachexia and signs of negligence on admission to hospital, it cannot be certain whether the parents followed the recommendations and provided optimal care at home. During these two months, the visits at the diabetological outpatient clinic were rescheduled due to the COVID-19 pandemic.
In the case of our patient, HHS developed in an uncommon manner as a complication of transient glucose metabolism disorders, which were most likely a result of the patient’s severe cachexia. Moreover, the parents failed to notice the patient’s polyuria and polydipsia and gave her fluids with high carbohydrate content, which probably contributed to the development of HHS. The amount of insulin produced in HHS is not sufficient to guarantee adequate glucose metabolism, especially after ingesting large amounts of carbohydrates [4]. The intake of these fluids resulted in an increase in osmolality, which accelerated the dehydration and loss of electrolytes, as described in other cases [10, 11].
As differential diagnosis of the transient hyperglycaemia stress-induced hyperglycaemia (SIH) should also be considered. It is a serious medical stage resulting from a traumatic injury, burn, surgery and critical or acute medical illness, requiring prompt treatment to decrease morbidity and mortality [12]. In a large American study of over 3.4 million patients, the prevalence of hyperglycaemia (> 180 mg/dl) was approximately 32% for intensive care and non-intensive care patients [13]. SIH usually resolves after recovery from acute medical trauma, the sooner the lighter the trauma [12]. SIH is caused by a combination of insufficient insulin secretion in response to hyperglycaemic effects of counter-regulatory hormones (glucagon, cortisol, epinephrine) and insulin resistance in later stage of diseases that have significant tissue damage [12]. In the case of our patient, normal level of C-reactive protein, mild leukocytosis (which could be the result of dehydration) and two-fold increase of procalcitonin above the reference value, do not allow to clearly identify a severe infection, as a cause of increase in glycaemia and the presence of HHS.
The primary manifestations of developing HHS – polyuria and polydipsia are highly difficult to identify in an adolescent with a severe disability and significantly limited contact. The patient could not communicate her needs and was completely dependent on the food and drink provided by the parents.
Cerebral palsy is a risk factor for malnutrition [14] which affects from 3.9 to 71% of such children [15]. A multicentre cross-sectional study in Turkey that investigated 1108 paediatric patients with cerebral palsy found that the prevalence of malnutrition was 57.2% according to the physicians’ clinical judgment [15]. The main causes of malnutrition identified by these authors, which also seem relevant to the presented case, were the insufficient knowledge of parents (46.2%), the lack of attention to the diet (24.1%) or inadequate time commitment (18.4%) [14]. An ambulatory nutritional network is currently developing in Poland, where such children and their parents can receive adequate education and support.
Glycaemic regulation disorders in patients with cachexia result from a significant increase of the secretion of insulin-contrary hormones – especially cortisol and catecholamines – leading to an acceleration of lipolysis and glycogenolysis [16]. The release of free fatty acids and glycerol results in an increase in insulin resistance and protein catabolism [16]. The products of lipolysis inhibit the action of insulin and glucose uptake by peripheral tissues (peripheral insulin resistance), increase liver gluconeogenesis and exhibit a toxic effect on pancreatic beta cells, resulting in their dysfunction (lipotoxicity) [16]. These mechanisms are described in the context of patients with advanced neoplastic diseases, but they also explain the development of HHS in an adolescent in the course of cachexia.
In accordance with the protocol in force in our department, diagnostic tests (including HbA1c, C-peptide, and levels of islet cell antibodies) are taken after the patient’s clinical condition has stabilized. Unfortunately, due to the severe and life-threatening cachexia, the patient was immediately transferred to the another department and the full diabetes diagnostic was postponed until a scheduled follow-up visit at the clinic. The patient did not live until the laboratory tests could be performed.
On the one hand she might have been in a very early stage of T1DM (pre-diabetes). It is also possible that our patient was close to developing malnutrition-related diabetes mellitus (MRDM) – she demonstrated 9 points per the diagnostic criteria by Bajaj (a result of 7–9 points is suggestive of MRDM, > 10 indicates MDRM) [17]. The Bajaj criteria include: age of onset of 10–30 years, leanness with BMI <19 kg/m2, history of malnutrition in childhood, stigmata of past or present malnutrition or deficiency state, moderate to severe hyperglycaemia, lack of proneness to ketosis in the absence of stress, insulin required to achieve metabolic control but no dependence on insulin for the prevention of ketosis and pancreatic calcification. MRDM is a rare type of diabetes associated with long-term malnutrition, often developing in early childhood, which mostly occurs in slim, malnourished men of poor socioeconomic status, aged 10 to 30 [17]. Undernutrition and bad dietary habits lead to the impairment of pancreatic beta cell functions, which is of key significance in the pathophysiology of this disease.
To the best of our knowledge, there are few recent reports regarding MRDM, with none concerning patients from developed countries. A mentioned above our patient had just started to develop glucose metabolism disorders and insulin resistance which, in the future, may have led to full MRDM. Insufficient insulin secretion by the pancreas resulted in lipolysis, whereas the ketones released from the adipose tissue lead to mild ketonemia. Due to the inability to independently control the intake of liquids, dehydration developed faster than normal. In addition, the intake of significant amounts of hyperosmolar sweet drinks resulted in HHS. The reason as to why the patient did not actually develop diabetes was most likely due to the rapid evolution of this severe condition. Insulin therapy decreased insulin resistance, therefore the patient did not require further insulin supply.
After a broad literature review, we consider this the first description of HHS that developed in the course of transient glucose metabolism disorders due to malnutrition. Additionally, the only other report we found that did not present HHS as a manifestation or complication of diabetes was a description of a 22-month-old Hispanic child with HHS developing as a consequence of severe gastroenteritis with dehydration and no subsequent evidence of T1DM [18].
Regarding the treatment of our patient, the administration of water for injection in the emergency room could be considered risky, because this hypotonic liquid, despite significant hyperosmolarity, could have led to a too rapid correction and subsequently to cerebral oedema and central pontine myelinolysis [19]. In order to prevent the rapid passage of water into the intracellular compartment, osmolality and sodium should be reduced gradually (< 3 mOsm/kg/H2O/hour and < 0.5 mmol/l/h) (Figure 1) [4, 6]. Rehydration is the most important part of HHS treatment.
HHS is associated with high mortality due to its complications, such as prerenal kidney failure, which were both present in our patient and reported by other authors [19]. The risk of thrombosis is greater than in DKA, due to the severe dehydration and hypertonicity that damage the endothelial cells [3]. The severe dehydration and hypertonicity may lead to the osmotic disruption of endothelial cells, resulting in the release of tissue factor and elevated vasopressin, which may contribute to the formation of blood clots [2]. Another problem that increases the mortality, also observed in our patient, is hypophosphataemia, which may result in rhabdomyolysis or paralysis and is associated with increased mortality in general. Therefore the patient received phosphate supplementation combined with calcium supplementation – in order to prevent the subsequent development of hypocalcaemia [4, 6]. Other reported complications associated with HHS include: pancreatitis, arrhythmia, cardiovascular collapse, cerebral oedema and malignant hyperthermia [11, 19].
The presented case demonstrates that HHS may pose many diagnostic difficulties and can be easily mistaken for new-onset diabetes. Given the high mortality and different treatment approach in HHS, a careful diagnosis for identifying HHS or DKA must always be carried out. Moreover, the case of our patient indicates that HHS can develop not only secondary to diabetes, but also in an adolescent in severe condition with transient blood glucose disorders, associated for example with extreme cachexia.

 ENGLISH
ENGLISH